
Tonifying-Qi-and-Detoxification Decoction attenuated injuries of colon and lung tissues in ulcerative colitis rat model via regulating NF-κB and p38MAPK pathway
Introduction
Ulcerative colitis (UC) is an intestinal disease dominated by non-specific inflammatory lesions of the rectum and colonic submucosa (1), as well as the entire colon and terminal ileum (2). The clinical characteristics of UC are persistent or recurrent diarrhea, stools with mucus blood or suppuration, abdominal pain, and various systemic symptoms (3). The disease is very difficult to cure and current treatment is mainly directed to symptom relief (1). Complications of UC include toxic megacolon, intestinal perforation, and lower gastrointestinal bleeding, which threaten the health of patients and cause them great anxiety (4). UC can also cause lung damage, resulting in a decrease of gas transfer and increase of residual volume: total lung capacity (RV:TLC) ratio (5,6). Recent years, with changes of dietary habits, the prevalence of UC has risen sharply.
Current treatments for UC include 5-aminosalicylic acid (5-ASA), sulfasalazine (SASP), glucocorticoids, immunomodulators, and biological agents (7,8), and although these drugs have some effect, more therapeutic strategies are required. Due to their multi-target, effectiveness, and safety, traditional Chinese herbal compounds are often used in the treatment of multiple human diseases, including UC (9,10), and have attracted increasing attention in the last few years. Zheng et al. (11) reported a clinical randomized controlled trial concerning traditional Chinese medicine combination therapy for patients with steroid-dependent UC. It is believed that herbal compounds not only obviously improve the symptoms of UC via promoting ulcer healing, but also reduce the adverse responses of Western medicine (10).
Tonifying-Qi-and-Detoxification Decoction (TQDD) is an herbal compound containing Glycyrrhiza uralensis Fisch, Angelica sinensis, Salvia miltiorrhiza Bunge, Houttuynia cordata Thunb, Scutellaria baicalensis Georgi, and Radix Sophora flavescens, which was established by an experienced practitioner of Chinese medicine, named Xinyue Wang. Earlier literatures reported that both Radix Astragali and Radix Angelicae Sinensis exerted excellent anti-inflammatory activity in multiple human diseases (12,13). More importantly, Yang et al. (14) discovered that both the Huangqi Jiegeng Decoction and Huangqi Huanglian Decoction exerted a predominant advantage in alleviating local inflammation and pathological damage of the lung and intestinal tract in UC. Based on the etiology and pathogenesis theory of Chinese medicine, tonifying Qi and activating blood are two important therapeutic principles in UC. However, until now, the efficacy and pharmacologic mechanism underlying the application of TQDD on UC remains unknown.
In the current research, colon mucosal tissue sensitization combined with TNBS-ethanol was used to establish UC rat model (6). By setting the SASP treatment as a positive control, the potential of TQDD in the treatment of UC was explored. The effects of SASP and TQDD treatment on UC-related lung damage were investigated, along with the internal mechanism related to the NF-κB pathway and p38MAPK pathway. This is the first study concerning the beneficial effects of TQDD on UC. The findings of our research will provide theoretical and experimental evidences for further probing the therapeutic potential of TQDD on UC. We present the following article in accordance with the ARRIVE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-892/rc).
Methods
Animal experiments
Experiments were performed under a project license (No. 2017024) granted by Laboratory Animal Ethics Committee of North China University of Science and Technology, in compliance with national guidelines for the care and use of animals. We purchased 96 healthy male Wistar rats (male, weighing 200±10 g) from Beijing Huafukang Biotechnology Co., Ltd. (Beijing, China) and housed them in our laboratory (25 ℃ 12 h night-dark cycle, free access to water and food) for 1 week to adapt to the new environment. Rats were then weighed and randomly divided into a negative control (NC) group, UC model group, sulfasalazine enteric-coated tablets (SASP) group, and TQDD group (low, middle, and high dosages) with 24 rats in each group. In addition, five healthy New Zealand white rabbits (male, weighing 2.5 kg) were purchased from Jinmuyang Laboratory Animal Breeding Co., Ltd. (Beijing, China).
Colon mucosal tissue sensitization combined with TNBS-ethanol stimulation was utilized to establish the UC rat model. Briefly, rabbits were sacrificed by air embolization, colon tissue was sampled, and mucosa tissue was isolated to mix with an equal volume of 0.9% saline solution. Following centrifugation (3,000 rpm, 30 min, 4 ℃), the supernatant was harvested to be re-suspended in the same volume of complete Freund’s adjuvant. Subsequently, the re-suspended solution was injected into the toes and groin of the rats (8 mg/each rat) on the 1st, 15th, and 22nd day, respectively. Rats were then fasted for 24 h while allowed free access to water before being anesthetized using Zoletil-50 (55 mg/kg) by intramuscular injection. TNBS-50% ethanol solution was then slowly placed into the colon (100 mg/kg) via the anus advanced to 8 cm proximal. The anus was kept high until rats awakened from anesthesia, and except for those in the NC group, all were subjected to stimulation. Moreover, SASP was prepared into aqueous solution after ultrasonic pulverization to 0.125 g/mL, and 1 mL SASP aqueous solution was given through the anus to rats in the SASP group. TQDD was decocted with deionized water twice, and the decoction was percolated and settled using ethanol extraction methods. For rats in the TQDD-low, middle, and high groups, 8.285, 16.57, and 33.14 g/kg (equal to twice the dosage for adults in clinical use) TQDD was given through the anus, respectively.
Following anaesthetization and sacrifice, the lung and colon tissues of rats were separated for subsequent experiments. Blood was harvested from abdominal aorta to separate serum, which was stored at −80 ℃.
Hematoxylin-eosin (HE) staining
Lung and colon tissues were placed in 4% paraformaldehyde solution (24 h, 4 ℃) and then embedded using paraffin and sliced into 6 µm sections. After de-paraffinizing in xylene solution and dehydration with a gradient ethanol solution, the sections were in turn dyed using hematoxylin solution, differentiated in 1% hydrochloric acid alcohol, dyed using eosin solution, and mounted with neutral resin. Results were observed under an optical microscope (Olympus, Japan).
Transmission electron microscope (TEM)
TEM was employed to observe the ultrastructure changes to alveolar epithelial type II cells (AEC-II), while isolation, culture, and verification of AEC II was performed as earlier described by Dobbs et al. (15) To observe the ultrastructure of AEC II in each group, samples were prepared as follows: AEC II was fixed into glutaraldehyde solution for 24 h, rinsed with PBS three times, fixed with 1% osmium acid for 1 h, rinsed with PBS, dehydrated with gradient ethanol solution, embedded using resin, sliced to 50 nm section, and dyed using uranyl acetate and lead citrate solution. Results were examined using a TEM (Olympus, Tokyo, Japan).
Quantitative reverse transcription PCR (qRT-PCR)
Total RNAs were collected using Trizol reagent. cDNA was synthesized with the help of a PrimeScriptTM RT Reagent Kit with a gDNA Eraser (Takara Biotechnology, Beijing, China) by using 2.0 µg total RNAs as temple. A SYBR Green qPCR kit (Beyotime Biotechnology, Shanghai, China) was used to test mRNA levels of nuclear factor-kappa B (NF-κB), NF-κB inhibitor α (IκBα), Bax, and Caspase 3 with GAPDH as an internal control. The primer’s sequences are illustrated in Table 1.
Table 1
| Name | Sequence (5'-3') |
|---|---|
| Bax | |
| Forward | TGCTTCAGGGTTTCATCCAG |
| Reverse | GGCGGCAATCATCCTCTG |
| Caspase 3 | |
| Forward | ACATGGCGTGTCATAAAATACC |
| Reverse | CACAAAGCGACTGGATGAAC |
| NF-κB | |
| Forward | GAAACCCTTTCTCTACTACCCC |
| Reverse | GATGCCTGTGTTGGATTTAGTG |
| IκBα | |
| Forward | CGTGTCTGCACCTAGCCTCTATC |
| Reverse | GCGAAACCAGGTCAGGATTC |
| GAPDH | |
| Forward | GACATGCCGCCTGGAGAAAC |
| Reverse | AGCCCAGGATGCCCTTTAGT |
qPCR, quantitative PCR; NF-κB, nuclear factor kappa B; IκBα, NF-κB inhibitor α.
Western blotting assay
Total proteins were collected by RIPA lysis buffer (Applygen, Beijing, USA) and detected by BCA kit (CW Biotech Co., Beijing, China). Proteins were then separated on SDS-PAGE gel and transferred to polyvinylidene fluoride (PVDF) membrane. Following blockage with 5% non-fat milk for 1 h, the PVDF membrane was incubated with anti-p38MAPK antibody (1:200, Santa Cruz Biotechnology, Santa Cruz, USA) or anti-β-actin antibody (1:1,000, Zhongshan Biotech, Beijing, China) overnight at 4 ℃. After washing with Tris buffer solution-Tween (TBST) solution, the PDVF membrane was incubated with IgG-HRP (1:1,000, Applygen, Beijing, China) for 2 h. Results were observed using enhanced chemiluminescence technique, and data were analyzed using Image-Pro Plus 6.0 software.
Immunohistochemistry (IHC) assay
IHC assay was carried out to assess activating transcription factor 2 (ATF2), c-jun, and c-fos expressions in lung and colon tissues. Briefly, 6 µm lung or colon sections were de-paraffinized in xylene solution and hydrated in a decreasing gradient ethanol solution. Sections were then treated with citrate antigen retrieval solution (Beyotime Biotechnology, 20 min, 95 ℃), permeabilized using 0.5% Triton X-100 solution (20 min), incubated with 3% H2O2 solution, and coated with 3% bovine saline albumin (BSA, Beyotime Biotechnology). Sections were then treated with anti-ATF2 antibody (1:250), anti-c-jun antibody (1:250), and anti-c-fos antibody (1:250, Abcam Biotechnology, MA, USA) overnight at 4 ℃ and HRP-conjugated secondary antibody for 60 min. Relative expressions were visualized using a diaminobenzidine (DAB) solution (Beyotime Biotechnology) and the cell nucleus was stained using hematoxylin solution. Results were observed under a microscope (Nikon, Japan).
Statistical analysis
Graphpad Prism 9.0 software was utilized for statistical analysis and results were exhibited as mean ± SD. One-way ANOVA was performed for gauging P values with a significance level of P<0.05.
Results
TQDD alleviated microstructure change of lung tissues in UC rat model
No rats died during experiment. After sacrifice, the lung and colon tissues of all rats were separated. There were no adverse events in each experimental group. HE staining was utilized to observe the microstructure of lung tissues. Figure 1A shows that while the lung structure of rats in the NC group was clear and without edema or fibrosis, there was considerable lymphocyte infiltration in the lung tissues of the model group. The pulmonary interstitial fibrous hyperplasia was arrested, and collagen proliferated around bronchi, small bronchi, and small vessels, which were surrounded by many collagen fibers, and extended to the pulmonary interstitium, characterized with sheets and bundles. The bronchial wall was thickened and deformed, and the vascular wall was thickened with some pulmonary alveoli fused to form bullae. In the SASP group, inflammatory cell infiltration was still obvious. Relative to the model group, the bronchial and vascular wall thickening was improved but fibrous hyperplasia around the airway was still severe. However, in the TQDD group, the degree of pulmonary consolidation and pulmonary fibrosis were significantly reduced compared with model and SASP groups, showing only a small amount of interstitial fibrous hyperplasia. The bronchial wall thickening was also significantly improved, and was most significant in the middle dose TQDD group. These results suggested that TQDD could alleviate microstructure change of lung tissues in UC rat model.
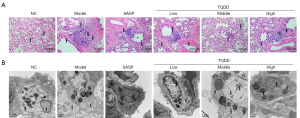
TQDD relived the ultrastructure damage of AEC-II in UC rat model
The ultrastructure of AEC-II in each group was then observed by TEM. As shown in Figure 1B, in the NC group, chromatin distribution in the nucleus was uniform, and organelles such as mitochondria and endoplasmic reticulum in the cytoplasm were morphologically normal with dense of lamellar bodies. While the villi were observed on the surface of the cell membrane, mitochondrial edema, endoplasmic reticulum expansion, perinuclear expansion, interstitial collagen proliferation, villus reduction, and an increase in cell volume were observed in the model group. Compared to the NC group, the main amelioration of AEC-II ultrastructure in TQDD groups were nuclear chromatin condensation, lamellar bodies decreased, and an interstitial collagen proliferation. Only a small amount of mitochondrial edema, endoplasmic reticulum dilatation, and interstitial collagen proliferation were observed in the low-dose TQDD group, while there was an occasional lamellar body vacuolation in the high-dose TQDD group. These results suggested that TQDD could relieve the ultrastructure damage of AEC-II in UC rat model.
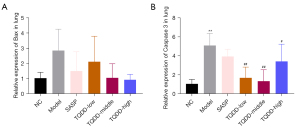
TQDD inhibited lung cell apoptosis in UC rat model
The lung cell apoptosis of each group was then evaluated via testing Bax and Capase 3 mRNA levels. As shown in Figure 2A,2B, relative to the NC group, both Bax and Caspase 3 expressions in lung tissues were raised (P<0.01 for Caspase 3 expression). Compared to the model group, SASP or TQDD treatment decreased the Bax and Caspase 3 mRNA levels in lung tissues (P<0.01 for Caspase 3 expression in TQDD-low and TQDD-middle groups, and P<0.05 for Caspase 3 expression in TQDD-high group). These outcomes suggested that TQDD could inhibit lung cell apoptosis in UC rat model.
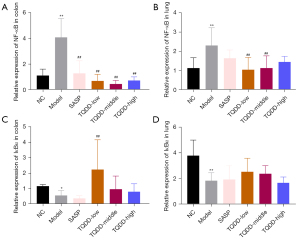
TQDD suppressed activation of NF-κB pathway in colon and lung tissues of UC rat model
NF-κB pathway is a key inflammation-related pathway in cells. Subsequent experiments were carried out to measure NF-κB and IκBα mRNA levels in colon and lung tissues. Results in Figure 3A,3B illustrated that NF-κB mRNA expressions in both colon and lung tissues were noticeably raised in the model group compared to the NC group (P<0.01). TQDD treatment lowered the NF-κB mRNA expressions in both colon and lung tissues (P<0.01), while SASP treatment only reduced it in colon tissues (P<0.01). However, the mRNA expressions of IκBα showed contrary phenomenon, and relative to the NC group, the IκBα mRNA expressions in both colon and lung tissues were notably lowered in the model group (Figure 3C,3D, P<0.05 or P<0.01). TQDD treatment enhanced the IκBα expressions in both colon and lung tissues (P<0.01 for colon tissues in the TQDD-low group), while SASP treatment had no significant effects in colon and lung tissues. These results suggested that TQDD could suppress the activation of NF-κB pathway in colon and lung tissues in UC rat model.
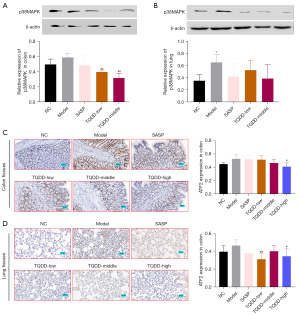
TQDD inhibited p38MAPK pathway in colon and lung tissues of UC rat model
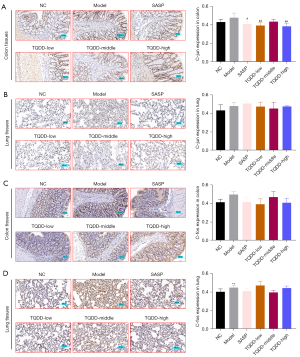
p38MAPK pathway was discovered to take part in UC development, and herein, its protein level in colon and lung tissues was assessed by Western blotting. Figure 4A,4B showed that relative to NC group, the p38MAPK protein level in lung tissue was increased in the model group (P<0.05). Moreover, compared to the model group, p38MAPK levels in colon and lung tissues were decreased in the TQDD group (P<0.01 for colon tissue in TQDD-low and middle groups). ATF2, c-jun, and c-fos were key downstream molecules of p38MAPK in cells. IHC was performed to evaluate their expressions in each group. Results showed that the expressions of ATF2, c-jun, and c-fos in colon and lung tissues were all increased in the model group, relative to the NC group (Figure 4C,4D, Figure 5A-5D, P<0.01 for c-fos expression in lung tissues), while SASP or TQDD treatment partially reversed their increased expression in colon and lung tissues (P<0.05 or P<0.01 as figure shown). These results suggested that TQDD could inhibit p38MAPK pathway in colon and lung tissues of UC rat model.
Discussion
As an inflammatory disease of the gastrointestinal tract, UC is very difficult to cure (1) and the World Health Organization considers it a Modern Difficult Disease (16). Recurrence of the disease is common and usually accompanied with parenteral lesions which mainly occur in the lungs (6). Previous literature reported that there was an association between UC and subclinical pulmonary abnormalities (17), which mainly manifested in the reduction of gas transfer and elevation of RV:TLC ratio (18). TQDD is a Chinese medicine compound, and we have shown it could alleviate lung structure injury and lung cell apoptosis in UC rat model. Moreover, TQDD suppressed the NF-κB and p38MAPK pathways in colon and lung tissues of UC rat model.
The pulmonary blood-gas barrier is a semi-permeable structure composed of the alveolar epithelial barrier, extracellular matrix, and pulmonary microvascular endothelial barrier, which are responsible for the exchange of oxygen and carbon dioxide in alveoli and blood (19,20). Integrity of the barrier is essential for pulmonary air exchange and preventing the reflux of substances in the blood into the interstitium and alveolar space (19). Disruption of the pulmonary blood-gas barrier leads to the entry of fluids, proteins, and inflammatory mediators into the alveolar space, which is the main pathological basis of lung injury (21,22). AEC-II are the main constituent cells of the pulmonary blood-gas barrier, which can synthesize and secrete pulmonary surfactant to repair lung tissue injury (23). In this research, we discovered that severe lung microstructure injury and cell apoptosis occurred in UC rat model and was accompanied by ultrastructure damage of AEC-II. TQDD treatment could improve the lung microstructure injury, cell apoptosis, and ultrastructure damage of AEC-II, showing it could exert a protective effect on UC-related lung injury.
NF-κB is a nuclear transcription factor in cells. As the convergence point of a variety of signal transduction pathways, it is not only involved in immune regulation, the inflammatory response, tumor development, and other physiological and pathological processes, but also participates in modulation of infection, cell cycle, cell differentiation, and apoptosis (24,25). NF-κB has been shown to be activated in UC, which can stimulate the secretion of multiple inflammatory cytokines to form an inflammatory cascade (26). Suppression of NF-κB was demonstrated to slow UC development (27), while activation was verified to promote UC progression (28). Previous literatures reported that some components of TQDD exerted excellent anti-inflammatory activity via regulating NF-κB pathway (29,30). For example, the glycyrrhetinic acid extracted from Glycyrrhiza uralensis Fisch was demonstrated to suppress NF-κB activation in TNF-α-induced hepatocytes (29). The polyhydroxyflavonoids extracted from Scutellaria baicalensis Georgi also exerted anti-inflammatory activity (30). Herein, we found NF-κB activity was enhanced both in colon and lung tissues of UC rat model, in which SASP or TQDD treatment suppressed the increase in NF-κB activity. Further, the expression of IKBα, the inhibitor of NF-κB, showed an opposite tendency. These findings show TQDD relieved colon and lung injuries in UC rat model by suppressing the NF-κB pathway.
MAPKs are important signaling systems for eukaryotic cells to mediate extracellular to intracellular responses (31). As a representative member of the MAPK family, p38MAPK exerts a critical regulatory influence on cellular inflammation, proliferation, stress, apoptosis, and tissue repair, which can be activated by various stresses, cell growth factors, and inflammatory cytokines (32). Downstream signaling proteins activated by p38MAPK transmit the stimulation signal to the nucleus and activate ATF2, c-jun, and c-fos to regulate its binding to transcription factor activating proteins, and affecting the transcriptional activity of DNA (33-35). Evidence confirms that p38MAPK pathways also participate in the pathogenesis of UC (36). Furthermore, earlier literature found that p38MAPK activation can directly activate IκB kinase, leading to phosphorylation of IκB protein, which in turn caused the activation of NF-κB (37). Moreover, earlier studies also found that some components of TQDD exerted excellent anti-inflammatory activity via regulating MAPK pathway (38,39). For example, Pu et al. (38) discovered that baicalein isolated from Scutellaria baicalensis Georgi could attenuate pancreatic inflammatory damage via modulating MAPK pathway. Li et al. (39) found that sophoraflavanone G extracted from Radix Sophora flavescens induced apoptosis of human leukemia cells through blocking MAPK activation. In this research, we found p38MAPK protein levels were raised in colon and lung tissues of UC rat model, which was accompanied by increases of ATF-2, c-jun, and c-fos expression. SASP or TQDD treatment reduced the p38MAPK protein level, as well as ATF-2, c-jun, and c-fos expression in colon and lung tissues. These findings show TQDD relieved colon and lung injuries of UC rat model also via suppressing the p38MAPK pathway.
In conclusion, this research confirmed the beneficial effect of TQDD on colon and lung tissues in UC rat model via regulating NF-κB and p38MAPK pathways. Considering the differences between groups in some results of our research were not statistically significant, more experiments are needed to further explore the effects of TQDD on UC in the future by altering its dosage. Moreover, whether there is an interaction between NF-κB and p38MAPK pathways in UC progression requires clarification. These findings may be helpful for finding effective interventions against the target and exploring therapeutic methods that can simultaneously protect and repair the lung-intestinal barrier in UC patients.
Acknowledgments
Funding: This study was supported by grants from the National Natural Science Foundation of China (No. 81704059), Natural Science Funds of Hebei Province (No. H2021209030), and Basic Innovation Team Project of Tangshan (No. 21130204D).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-892/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-892/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-892/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Approval was obtained from the Laboratory Animal Ethics Committee of North China University of Science and Technology prior to the study (No. 2017024). Experiments were carried out in compliance with national guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ghosh S, Shand A, Ferguson A. Ulcerative colitis. BMJ 2000;320:1119-23. [Crossref] [PubMed]
- Călin R, Nuta P. Therapeutic Management in Ulcerative Colitis. Internal Medicine 2018;15:61-72. [Crossref]
- Yang X, Yao W, Liu W, et al. Clinical manifestations and outcomes in severe ulcerative colitis. Front Med China 2007;1:192-5. [Crossref] [PubMed]
- Lichtenstein G, Shahabi A, Seabury S, et al. Complications of Crohnʼs Disease and Ulcerative Colitis: Understanding the Lifetime Risks: 716. American Journal of Gastroenterology 2017;112:S395-6. [Crossref]
- Marvisi M, Borrello PD, Brianti M, et al. Changes in the carbon monoxide diffusing capacity of the lung in ulcerative colitis. Eur Respir J 2000;16:965-8. [Crossref] [PubMed]
- Liu Y, Wang XY, Yang X, et al. Lung and intestine: a specific link in an ulcerative colitis rat model. Gastroenterol Res Pract 2013;2013:124530. [Crossref] [PubMed]
- Seo GS, Chae SC. Biological therapy for ulcerative colitis: an update. World J Gastroenterol 2014;20:13234-8. [Crossref] [PubMed]
- Zhao X, Zhou C, Ma J, et al. Efficacy and safety of rectal 5-aminosalicylic acid versus corticosteroids in active distal ulcerative colitis: a systematic review and network meta-analysis. Sci Rep 2017;7:46693. [Crossref] [PubMed]
- Rezayat F, Hashempur MH, Tavahen H, et al. The efficacy of Ramak (a traditional herbal medicine preparation) for patients with ulcerative colitis: a pilot, randomized, triple-blinded, placebo-controlled clinical trial. Eur J Integr Med 2020;39:101209. [Crossref]
- Shen R, Liu M, Zhu XD, et al. Experimental research progress on traditional Chinese medicine treatments for ulcerative colitis. Chinese Traditional and Herbal Drugs 2018;49:1721-5.
- Zheng K, Shen H, Jia J, et al. Traditional Chinese medicine combination therapy for patients with steroid-dependent ulcerative colitis: study protocol for a randomized controlled trial. Trials 2017;18:8. [Crossref] [PubMed]
- Li WK, Wang GF, Wang TM, et al. Protective effect of herbal medicine Huangqi decoction against chronic cholestatic liver injury by inhibiting bile acid-stimulated inflammation in DDC-induced mice. Phytomedicine 2019;62:152948. [Crossref] [PubMed]
- Deng P, Li X, Wei Y, et al. The herbal decoction modified Danggui Buxue Tang attenuates immune-mediated bone marrow failure by regulating the differentiation of T lymphocytes in an immune-induced aplastic anemia mouse model. PLoS One 2017;12:e0180417. [Crossref] [PubMed]
- Yang X, Wang XY, Jing S, et al. Effect of treatment from the lung and treatment from the intestine on vasoactive intestinal peptide contents of ulcerative colitis rats: a comparison study. Zhongguo Zhong Xi Yi Jie He Za Zhi 2015;35:222-7. [PubMed]
- Dobbs LG. Isolation and culture of alveolar type II cells. Am J Physiol 1990;258:L134-47. [PubMed]
- Ray C, Sagar P. Management of Crohn’s disease and ulcerative colitis. Surgery (Oxford) 2020;38:318-21. [Crossref]
- Songür N, Songür Y, Tüzün M, et al. Pulmonary function tests and high-resolution CT in the detection of pulmonary involvement in inflammatory bowel disease. J Clin Gastroenterol 2003;37:292-8. [Crossref] [PubMed]
- Keely S, Talley NJ, Hansbro PM. Pulmonary-intestinal cross-talk in mucosal inflammatory disease. Mucosal Immunol 2012;5:7-18. [Crossref] [PubMed]
- Hachenberg T, Rettig R. Stress Failure of the Blood-Gas Barrier. Curr Opin Anaesthesiol 1998;11:37-44. [Crossref] [PubMed]
- Kuehn A, Kletting S, de Souza Carvalho-Wodarz C, et al. Human alveolar epithelial cells expressing tight junctions to model the air-blood barrier. ALTEX 2016;33:251-60. [Crossref] [PubMed]
- Peng LY, Yuan M, Shi HT, et al. Protective Effect of Piceatannol Against Acute Lung Injury Through Protecting the Integrity of Air-Blood Barrier and Modulating the TLR4/NF-κB Signaling Pathway Activation. Front Pharmacol 2019;10:1613. [Crossref] [PubMed]
- Gupta RC, Pitt JE, Zaja-Milatovic S. Blood-Brain Barrier Damage and Dysfunction by Chemical Toxicity. Handbook of Toxicology of Chemical Warfare Agents (Second Edition) 2015:725-39.
- Mühlfeld C, Neves J, Brandenberger C, et al. Air-blood barrier thickening and alterations of alveolar epithelial type 2 cells in mouse lungs with disrupted hepcidin/ferroportin regulatory system. Histochem Cell Biol 2019;151:217-28. [Crossref] [PubMed]
- Pflug KM, Sitcheran R. Targeting NF-κB-Inducing Kinase (NIK) in Immunity, Inflammation, and Cancer. Int J Mol Sci 2020;21:8470. [Crossref] [PubMed]
- Scott O, Roifman C. NF-kB Pathway and the Goldilocks Principle: Lessons from Human Disorders of Immunity and Inflammation. J Allergy Clin Immunol 2019;143:1688-701. [Crossref] [PubMed]
- Yue B, Ren J, Yu Z, et al. Pinocembrin alleviates ulcerative colitis in mice via regulating gut microbiota, suppressing TLR4/MD2/NF-κB pathway and promoting intestinal barrier. Biosci Rep 2020;40:BSR20200986. [Crossref] [PubMed]
- Yang QY, Ma LL, Zhang C, et al. Exploring the Mechanism of Indigo Naturalis in the Treatment of Ulcerative Colitis Based on TLR4/MyD88/NF-κB Signaling Pathway and Gut Microbiota. Front Pharmacol 2021;12:674416. [Crossref] [PubMed]
- Zhu H, Huang S, Yue M, et al. Dysregulated Up-Frameshift Protein 1 Promotes Ulcerative Colitis Pathogenesis Through the TNFR1-NF-κB/MAPKs Pathway. Dig Dis Sci 2018;63:2593-603. [Crossref] [PubMed]
- Chen HJ, Kang SP, Lee IJ, et al. Glycyrrhetinic acid suppressed NF-κB activation in TNF-α-induced hepatocytes. J Agric Food Chem 2014;62:618-25. [Crossref] [PubMed]
- Huang WH, Lee AR, Yang CH. Antioxidative and anti-inflammatory activities of polyhydroxyflavonoids of Scutellaria baicalensis GEORGI. Biosci Biotechnol Biochem 2006;70:2371-80. [Crossref] [PubMed]
- Lake D, Corrêa SA, Müller J. Negative feedback regulation of the ERK1/2 MAPK pathway. Cell Mol Life Sci 2016;73:4397-413. [Crossref] [PubMed]
- Cuadrado A, Nebreda AR. Mechanisms and functions of p38 MAPK signalling. Biochem J 2010;429:403-17. [Crossref] [PubMed]
- Zhou H, Cai L, Zhang X, et al. ARHGEF39 promotes tumor progression via activation of Rac1/P38 MAPK/ATF2 signaling and predicts poor prognosis in non-small cell lung cancer patients. Lab Invest 2018;98:670-81. [Crossref] [PubMed]
- Hong IK, Kim YM, Jeoung DI, et al. Tetraspanin CD9 induces MMP-2 expression by activating p38 MAPK, JNK and c-Jun pathways in human melanoma cells. Exp Mol Med 2005;37:230-9. [Crossref] [PubMed]
- Chun SY, Kim S, Nam KS, et al. Anti-metastatic potential of a proton beam is regulated by p38 MAPK/c-Fos signaling pathway in TPA-treated HepG2 human hepatocellular carcinoma. Biomed Pharmacother 2018;99:904-12. [Crossref] [PubMed]
- Zhao X, Kang B, Lu C, et al. Evaluation of p38 MAPK pathway as a molecular signature in ulcerative colitis. J Proteome Res 2011;10:2216-25. [Crossref] [PubMed]
- Korus M, Mahon GM, Cheng L, et al. p38 MAPK-mediated activation of NF-kappaB by the RhoGEF domain of Bcr. Oncogene 2002;21:4601-12. [Crossref] [PubMed]
- Pu WL, Bai RY, Zhou K, et al. Baicalein attenuates pancreatic inflammatory injury through regulating MAPK, STAT 3 and NF-κB activation. Int Immunopharmacol 2019;72:204-10. [Crossref] [PubMed]
- Li ZY, Huang WC, Tu RS, et al. Sophoraflavanone G Induces Apoptosis in Human Leukemia Cells and Blocks MAPK Activation. Am J Chin Med 2016;44:165-76. [Crossref] [PubMed]
(English Language Editor: B. Draper)


