
Pralsetinib for the treatment of a RET-positive advanced non-small-cell lung cancer patient harboring both ANK-RET and CCDC6-RET fusions with coronary heart disease: a case report
Introduction
Rearranged during transfection (RET) fusion are found in several solid tumors, and the incidence of RET fusion is approximately 1–2% in non-small-cell lung cancer (NSCLC) (1-3). Search with the keywords “RET rearrangement, NSCLC, coexistance” with no data has been reported in PubMed database. These lung cancer patients mostly were adenocarcinoma, younger, no smoking, and prone to brain metastasis (4,5). The fusion of RET with chaperone genes encodes a chimeric tyrosine kinase, which forms a protein dimerization domain inside the cell and generates a ligand-independent activation of the intracellular RET tyrosine kinase domain. Then RET can be abnormally expressed in normally transcriptionally silenced cells, thereby activating the mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K)/retrovirus from AKR mice (AKT) signaling pathway being activated, which drives tumorigenesis and development of tumors (6). The most common fusion partners in NSCLC are kinesin family member 5B (KIF5B) and coiled-coil domain-containing protein 6 (CCDC6) (7).
Regardless of the type of fusion partner, RET fusion-positive NSCLC has a therapeutic response to RET tyrosine kinase inhibitors (8). Based on two global phase II clinical trials [ARROW (9) and LIBRETTO-001 (10)], the U.S. Food and Drug Administration (FDA) approved pralsetinib and selpercatinib to treat RET fusion-positive advanced NSCLC in 2020. These two drugs were soon included in the National Comprehensive Cancer Network (NCCN) guidelines and became preferred first-line treatments for RET fusion-positive advanced NSCLC (8). The multikinase inhibitors cabozantinib and vandetanib have limited efficacy against RET targets (8). If RET fusion is found during first-line systemic treatment, the original planned treatment can be continued or terminated, followed by either of the preferred drugs pralsetinib and selpercatinib or the choice of cabozantinib (8).
Notably, pralsetinib was introduced to Boao Lecheng, the only zone in China that can use the latest drugs and devices without approvement of NMPA, the same month (September 4, 2020) after pralsetinib approved by U.S. FDA. Pralsetinib’s global prescription is highly synchronized, so Chinese patients and American patients can receive effective treatment programs simultaneously.
This paper reports the regression of NSCLC in a patient with dual-RET-fusion advanced NSCLC combined with coronary heart disease after pralsetinib treatment before it came to market in China. We present the following case in accordance with the CARE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1237/rc).
Case presentation
The patient was a 64-year-old male with an 8-year history of coronary heart disease. He had irregular anti-platelet aggregation and received blood lipid regulation treatment (the specifics were unknown). He did not have a smoking history and sought treatment due to chest tightness and shortness of breath for more than 2 weeks. On October 15, 2019, the chest CT diagnosis indicated left upper lobe tumor (1.6 cm × 1.6 cm), left-sided hydropneumothorax, multiple nodules in both lungs, and pleural metastasis. The tumor, node, metastasis stage was cT4N0M1 stage IV. The diagnosis of lung adenocarcinoma was confirmed by analysis of left pleural fluid cytology specimens combined with hematoxylin and eosin staining for cytological morphology analysis and analysis of thyroid transcription factor 1 (TTF-1) (+), aspartate protease A (Napsin A) (+), P40 (−), and p53 (−) expression by immunohistochemistry. Next-generation sequencing (NGS) was used to perform DNA-based panel sequencing of 520 tumor-associated genes in cytology specimens (Burning Rock OncoScreen PlusTM). The results showed that there were two RET fusions in the patient, ANK3 (ankyrin)-RET (C1: R12), with a mutation abundance of 14.00%, and CCDC6-RET (A43: R12), with a mutation abundance of 7.63%.
From October 30, 2019, to March 24, 2020, pemetrexed + nedaplatin + bevacizumab was given for six cycles. From April 24, 2020 to August 17, 2020, four cycles of pemetrexed + bevacizumab maintenance treatment were given, and the progression-free survival (PFS) of the patient after receiving first-line platinum-based chemotherapy was 10 months.
The CT scan on October 29, 2020 showed that the tumor size was 1.2 cm × 1.2 cm, but his carcinoembryonic antigen was continuously increasing. The treatment was switched to alectinib from October 29, 2020 to January 20, 2021. The CT scan on January 20, 2021, showed that the left lung tumor was approximately 3.4 cm × 3.7 cm; the left pleural effusion was more than before, so he was diagnosed with disease progression. The treatment was changed to anlotinib from January 21, 2021, to February 2, 2021, which drug was discontinued for grade 2 proteinuria.
After the review of our application under the Interim Measures for the Management and Use of Urgently Imported Drugs in the Boao Lecheng International Medical Tourism Pilot Zone of Hainan, the patient was granted the right to take the urgently needed drug pralsetinib. And a written informed consent was obtained from the patient for this treatment. On February 18, 2021, 400 mg of pralsetinib was administered once a day (QD) as fourth-line treatment. The CT (baseline period) before starting the treatment of pralsetinib is shown in Figure 1A. On February 23, 2021, the drug was interrupted due to acute myocardial infarction, and anticoagulation and antiplatelet aggregation treatment was given due to his refusal of stent placement. After discussion by the multidisciplinary team, it was concluded that the acute myocardial infarction was not related with pralsetinib. Therefore, the drug was resumed on March 4, 2021 at the original dose of 400 mg QD. On March 9, 2021, the drug was interrupted again due to acute coronary syndrome, and percutaneous balloon coronary dilatation of the diseased vessels was performed. The attending physician considered that the acute coronary syndrome was caused by the underlying cardiac disease of the patient and had little correlation with pralsetinib. On April 3, 2021, the medication was resumed again at 400 mg QD. The CT scan (Figure 1B) on May 23, 2021, showed that the size of tumor was significantly smaller. According to the evaluation criteria of the World Health Organization (WHO), the tumor was reduced by 79% and could be assessed as partial remission (PR). According to the Response Evaluation Criteria in Solid Tumors (RECIST) criteria, the tumor volume shrank by 17%, which can be considered stable disease. The CT scan (Figure 1C) on August 13, 2021 suggested that the tumor had continued to shrink (Figure 2), achieved PR in RECIST 1.1 as well.
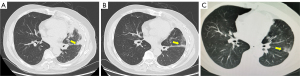
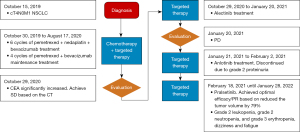
Overall, the safety of pralsetinib was manageable, and the adverse events included grade 2 leukopenia, grade 2 neutropenia, and grade 3 erythropenia. The patient developed dizziness and fatigue, which were relieved after infusion of suspended red blood cells. Currently, the patient continues to receive 400 mg of pralsetinib (QD). As of January 28, 2022, the last follow-up time, he is still in continuous remission and the treatment is ongoing.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
This paper reports a rare case of a dual-RET-fusion NSCLC patient with coronary heart disease who received fourth-line treatment of 400 mg pralsetinib (QD). Pralsetinib treatment was interrupted twice due to acute myocardial infarction and acute coronary syndrome. After drug and interventional treatment, the original dose was restored to achieve PR. He is still under treatment and is still in remission as of August 31, 2021.
With the development of molecular technology and molecular-targeted drugs, the treatment pattern of advanced lung cancer has undergone tremendous changes, shifting from chemotherapy-focused regimens to specific treatment strategies that target positive driver genes. With the continuous evolution of treatment models, advanced lung cancer is expected to become a manageable chronic disease.
As one of the rare gene mutations in NSCLC, RET only started to be detected relatively late in China, and the lack of unified standards among the various molecular pathology laboratories affected the identification of NSCLC populations carrying RET fusion mutations. In June 2021, the Expert Consensus on Clinical Practice of RET Fusion Detection in Non-Small-Cell Lung Cancer in China was published. It recommended selecting a reasonable detection method based on the type, quantity, and quality of the specimens, the type and quantity of the gene mutations to be examined, and the laboratory conditions. NGS for DNA/RNA is the preferred platform, and multiplatform detection and verification should be performed when necessary (7).
In 2011, researchers discovered the first fusion mutation of the RET gene encoding the receptor tyrosine kinase in lung adenocarcinoma tissue (11). NSCLC carrying RET fusion benefits little from traditional chemotherapy, multikinase inhibitors, or immunotherapy (8). In 2020, a retrospective multicenter analysis of 45 Chinese patients showed that the PFS of patients receiving chemotherapy (n=29), multikinase inhibitors (n=19), or immune checkpoint inhibitors (n=11) was 3.5, 3.8, and 2.5 months. The overall response rate (ORR) was 20% among the 10 patients who received immune checkpoint inhibitor therapy (12). In view of the low survival benefit and possible toxicity, only some chemotherapy regimens and multikinase inhibitors are recommended, as nonpreferred treatments (8).
Pralsetinib is a potent and highly selective RET TKI with fewer off-target effects. ARROW was a clinical trial to evaluate the efficacy of pralsetinib against RET-mutated solid tumors, including a phase I dose escalation cohort and a phase II dose expansion cohort. The results of that study confirmed the excellent safety and efficacy of pralsetinib as the first- or second-line treatment of RET fusion-positive NSCLC [first-line ORR 79%, median PFS 13.0 months (n=68)] (9). The amendment expanded inclusion criteria to allow recruitment of treatment-naive patients eligible for standard platinum-based therapy who had previously not been permitted. The ORR was 88%, and the median PFS had not yet been reached in this part (n=25). The ORR of patients receiving second-line platinum-containing treatment was 62%, and the median PFS was 16.5 months (n=126) (9). According to the World Conference on Lung Cancer report in 2021, the data of pralsetinib in the Chinese subgroup are consistent with those of the global group. Among the patients who had previously platinum-based chemotherapy, the ORR was 66.7%. and the ORR was 80% in the treatment-naive patients (13).
Based on ARROW data, the FDA approved pralsetinib as the first- or second-line treatment of RET fusion-positive advanced NSCLC patients on September 4, 2020. On March 24, 2021, the National Medical Products Administration of China granted conditional marketing approval for application of pralsetinib to adult locally advanced or metastatic NSCLC patients positive for RET fusion who had received prior platinum-containing chemotherapy.
At the time of treatment of this patient, pralsetinib had not been brought to market in China. According to the Interim Measures for the Management and Use of Urgently Imported Drugs in the Boao Lecheng International Medical Tourism Pilot Zone of Hainan, due to urgent clinical needs, drugs that have been approved for marketing in the United States, the European Union, Japan, and other countries or regions but have not been approved for registration in China or cannot be replaced by domestic registered products can be imported and legally used in the pilot zone. On September 14, 2020, pralsetinib was used for the first time in Boao Lecheng. The patient in this study received pralsetinib treatment after the failure of front-line treatment. The first follow-up CT showed that the tumor was 79% smaller than it was at baseline (based on the WHO criteria) or 17% smaller (based on the RECIST criteria). The data of pralsetinib in the Chinese subgroup, except for the CR cases, the maximum reduction of the tumor shrinkage was observed is 80%. Otherwise, there were not dual-RET-fusion advanced NSCLC case reported in the Chinese subgroup (13). The clinical antitumor activity of pralsetinib against RET-fusion NSCLC was confirmed. Given that pralsetinib mildly inhibits the Janus kinase 2 pathway, it is necessary to pay attention to the bone marrow suppression effect in clinical practice. The leukopenia, neutropenia, and erythropenia occurring in this patient were grade 2 or less.
Based on the discussion of this case of treating a rare dual-RET-fusion NSCLC patient with pralsetinib, we believe that the pralsetinib can effectively control the tumor development of patients with two RET fusions. For well-controlled cardiovascular lesions, even severe myocardial infarction, pralsetinib also has therapeutic benefits, which can provide a reference value for future complicated comorbidities. In addition, the patients in this case report still benefited from pralsetinib when they resumed medication nearly 40 days after interrupted.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1237/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1237/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Platt A, Morten J, Ji Q, et al. A retrospective analysis of RET translocation, gene copy number gain and expression in NSCLC patients treated with vandetanib in four randomized Phase III studies. BMC Cancer 2015;15:171. [Crossref] [PubMed]
- Michels S, Scheel AH, Scheffler M, et al. Clinicopathological Characteristics of RET Rearranged Lung Cancer in European Patients. J Thorac Oncol 2016;11:122-7. [Crossref] [PubMed]
- Li W, Guo L, Liu Y, et al. Potential Unreliability of Uncommon ALK, ROS1, and RET Genomic Breakpoints in Predicting the Efficacy of Targeted Therapy in NSCLC. J Thorac Oncol 2021;16:404-18. [Crossref] [PubMed]
- Cong XF, Yang L, Chen C, et al. KIF5B-RET fusion gene and its correlation with clinicopathological and prognostic features in lung cancer: a meta-analysis. Onco Targets Ther 2019;12:4533-42. [Crossref] [PubMed]
- Drilon A, Lin JJ, Filleron T, et al. Frequency of Brain Metastases and Multikinase Inhibitor Outcomes in Patients With RET-Rearranged Lung Cancers. J Thorac Oncol 2018;13:1595-601. [Crossref] [PubMed]
- Arighi E, Borrello MG, Sariola H. RET tyrosine kinase signaling in development and cancer. Cytokine Growth Factor Rev 2005;16:441-67. [Crossref] [PubMed]
- Molecular pathology cooperation group of Tumor Pathology Committee of China Anti Cancer Association, Molecular Pathology Group of Pathology Branch Of Chinese Medical Association, National Pathology Quality Control Center. Expert consensus on clinical detection of RET gene fusion in non-small cell lung cancer in China. Chinese Journal of Pathology 2021;50:583-91.
- National Comprehensive Cancer Network® (NCCN®). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Non-Small Cell Lung Cancer. Version 5. 2021-June 15, 2021.
- Curigliano G, Gainor J F, Griesinger F, et al. Safety and efficacy of pralsetinib in patients with advanced RET fusion-positive non-small cell lung cancer: Update from the ARROW trial. J Clin Oncol 2021;39:9089. [Crossref]
- Besse B, Drilon AE, Solomon BJ, et al. Updated overall efficacy and safety of selpercatinib in patients (pts) with RET fusion+ non-small cell lung cancer (NSCLC). J Clin Oncol 2021;39:9065. [Crossref]
- Ju YS, Lee WC, Shin JY, et al. A transforming KIF5B and RET gene fusion in lung adenocarcinoma revealed from whole-genome and transcriptome sequencing. Genome Res 2012;22:436-45. [Crossref] [PubMed]
- Lu C, Dong XR, Zhao J, et al. Association of genetic and immuno-characteristics with clinical outcomes in patients with RET-rearranged non-small cell lung cancer: a retrospective multicenter study. J Hematol Oncol 2020;13:37. [Crossref] [PubMed]
- Zhou Q, Wu YL, Chang JH, et al. Efficacy and Safety of Pralsetinib in Chinese Patients with Advanced RET Fusion+ Non-Small Cell Lung Cancer. Presented at: 2021 World Conference on Lung Cancer; September 8-14, 2021. Virtual. Abstract M02.02.


