
The diagnostic value of endoplasmic reticulum stress-related specific proteins GRP78 and CHOP in patients with sepsis: a diagnostic cohort study
Introduction
Sepsis is defined as life-threatening organ dysfunction caused by the host’s defective response to infections (1). Sepsis is a serious complication of acute and critical clinical patients. It is often induced by infections, severe trauma, burns, major operations, and other factors, and can lead to septic shock and multiple organ failure. With the aging of the population, the incidence of sepsis is also rising, bringing great burden to the society. The key to the treatment of sepsis is early recognition and accurate assessment, followed by appropriate intervention. Although there have been advances in diagnosis and treatment, the incidence of sepsis is still escalating yearly. In 2015, the number of deaths related to sepsis in China was 1,025,997. Globally, the annual number of sepsis cases is as high as 31 million cases, with a mortality rate of 30% to 50%, and therefore, this represents a significant global medical problem (2,3). The European Society of Critical Care Medicine (ESICM) and the American Society of Critical Care Medicine (SCCM) jointly published the Guidelines for Sepsis 3.0 in 2016, which emphasized the importance of early diagnosis and evaluation of the disease. However, the existing observation and evaluation indexes are often inadequate. Although C-reactive protein (CRP), interleukin (IL)-6, procalcitonin (PCT), and lactic acid (LAC) play an important role in clinical applications, relevant reviews have shown that it is often difficult to achieve the balance between sensitivity and specificity. Despite PCT’s higher specificity for bacterial infection, its level can also be elevated in non-infected conditions (4,5). False-positive results could lead to a misdiagnosis with crucial consequences for the treatment. Patients might be exposed to adverse effects and resistance to antibiotics if antimicrobials are used inappropriately. Recently, a previous study has demonstrated that the protein levels of CHOP and GRP78, markers of endoplasmic reticulum stress (ERS) activation, increased rapidly and continuously in the serum of patients with sepsis (6), suggesting that these two proteins may play an important role in the occurrence and development of sepsis. It is reported the transcription factors CHOP and GRP78 induced by ERS could contribute to sepsis-induced apoptosis (7). Therefore, this study evaluated the role of GRP78 and CHOP in the diagnosis of septic patients by measuring the serum levels of these ERS-related specific proteins. We present the following article in accordance with the STARD reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1445/rc).
Methods
Study design and setting
A total of 52 patients with sepsis infection and 26 patients with non-sepsis infection, who were admitted to the Department of Intensive Care Unit of Shanghai East Hospital and the Department of Anaesthesia, Critical Care and Pain Medicine of Fudan university Shanghai Cancer Center from February 1, 2018 to September 30, 2018, were enrolled in this study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This research project was approved by the Ethics Committee of Shanghai East Hospital and Shanghai Cancer Center, Fudan University, and was implemented with the informed consent of the patients.
Definition and grouping
Participants aged ≥18 years and <80 years were included. Patients admitted to our ICU during the study period who met the 2016 SCCM/ ESICM Sepsis 3.0 diagnostic criteria were allocated to the sepsis infected group. Patients were enrolled into the non-sepsis infected group if they satisfied the following inclusion criteria: (I) patients presented with symptoms of systemic inflammatory response syndrome (SIRS) and a clear infection site; (II) no new symptoms of dysfunction of any organ were present; and (III) patients did not meet the Sepsis 3.0 diagnostic criteria. The following exclusion criteria were applied: (I) patients in the terminal stages of malignant tumors; (II) patients with a clear diagnosis of acute coronary syndrome or patients with cerebral infarction; (III) patients with an expected survival time of less than 48 h. If patients in group II showed aggravation of infection during the course of disease and met the diagnosis of sepsis, they would be excluded from this group and transferred to group I.
Data collection
The basic patient characteristics including age, gender, daily Sequential Organ Failure Assessment (SOFA) score and Acute Physiology and Chronic Health Evaluation II (APACHE II) score, disease characteristics and survival information, and baseline laboratory tests were collated. A total of 4 mL venous blood was collected on days 1, 2, 3, and 7 following admission to the ICU. The serum was centrifuged within 15 min and stored at −80 ℃ for GRP78 and CHOP concentration detection. The levels of GRP78 and CHOP in the serum supernatant were detected using ELISA kits (Human CHOP ELISA Kit, MLBio) according to the manufacturer’s instructions. Each specimen had two duplicate holes, and the mean value was taken. The experiment was repeated 6 times. Serum CRP, IL-6, PCT, and LAC levels were detected on the day of blood collection. Patients were shortly follow-up for the 28-day mortality.
Statistical analyses
Statistical analysis was performed using SPSS 19.0 software and independent sample t-tests were used for comparison between the two groups. Receiver operating characteristic (ROC) curves and the area under the ROC curve (AUC) were used to evaluate the accuracy of biomarkers in the diagnosis of sepsis. To study the impact of the combination of GRP78 and CHOP for diagnosis, ROC curves analysis was based on binary logistic regression. A two-sided P value <0.05 was considered statistically significant.
Results
The CRP, PCT, APACHE II, and SOFA of group I patients were higher than those of group II patients. Age, IL-6, and LAC between two groups have no significant difference (P>0.05; Table 1). The serum GRP78 and CHOP levels at day 1 in group I were higher than those in group II (P<0.001; Table 2). Furthermore, the mortality rate of sepsis infected patients (group I) was higher than that of non-septic patients (group II; Table 1), with the outcome at day 28 in the ICU as the end point.
Table 1
| Group | Septic patients | Non-septic patients | P values |
|---|---|---|---|
| Number of patients | 52 | 26 | |
| Age (years) | 71.27±13.50 | 67.08±18.18 | 0.254 |
| APACHE II score | 16.13±6.03 | 10.77±4.69 | <0.001 |
| SOFA score | 7.58±3.84 | 0.054±0.51 | <0.001 |
| CRP (mg/L) | 130.26±53.29 | 70.35±50.29 | <0.001 |
| IL-6 (ng/L) | 418.82±1,044.18 | 129.48±240.01 | 0.168 |
| PCT (mg/L) | 37.14±37.68 | 3.01±8.74 | <0.001 |
| LAC (mmol/L) | 2.43±2.21 | 1.57±1.39 | 0.073 |
| 28-day mortality (survival/death) | 36/16 | 24/2 | 0.023 |
APACHE II, Acute Physiology and Chronic Health Evaluation II; SOFA, Sequential Organ Failure Assessment; CRP, C-reactive protein; IL, interleukin; PCT, procalcitonin; LAC, lactic acid.
Table 2
| Indexes | Septic infected group | Non-septic infected group | P values |
|---|---|---|---|
| GRP78 (ng/mL) | 208.79±21.76 | 190.55±46.87 | 0.021 |
| CHOP (ng/mL) | 6.56±0.69 | 5.81±0.57 | <0.001 |
GRP78, glucose regulated protein 78; CHOP, C/EBP homologous protein.
ROC curve analysis of GRP78 and CHOP as diagnostic markers in group I and group II were performed at days 1, 2, 3, and 7 (Table 3; Figures 1,2). On day 2 of admission to ICU, the GRP78 levels showed the best efficacy at diagnosing sepsis, with a cut-off value of 157.29 ng/mL (sensitivity, 75.0%; specificity, 73.1%) (P<0.05). On day 2 of admission to ICU, CHOP levels also showed the highest efficacy at diagnosing sepsis, with a cut-off value of 4.951 ng/mL (sensitivity, 57.7%; specificity, 96.2%) (P<0.05).
Table 3
| Number of days in the ICU | GRP78 | CHOP | |||
|---|---|---|---|---|---|
| AUC | P values | AUC | P values | ||
| Day 1 | 0.691 | 0.006 | 0.780 | <0.001 | |
| Day 2 | 0.771 | 0.000 | 0.813 | <0.001 | |
| Day 3 | 0.680 | 0.010 | 0.507 | 0.916 | |
| Day 7 | 0.714 | 0.002 | 0.660 | 0.022 | |
GRP78, glucose regulated protein 78; CHOP, C/EBP homologous protein; AUC, area under the curve; ICU, intensive care unit.
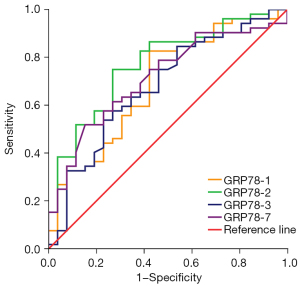
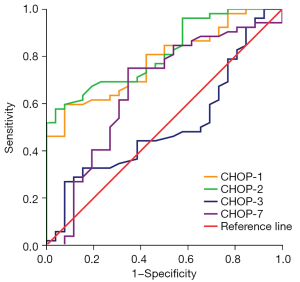
The ROC curve comparisons demonstrated the diagnostic ability of each indicator for sepsis (Figure 3; Table 4). The results showed that the maximum AUC of PCT, and CRP was 0.910, and 0.802, respectively. When GRP78 was used as a biomarker in the diagnosis of sepsis, the AUC under the ROC curve (0.771) (95% CI: 0.662–0.880) was higher than that of IL-6, and LAC (0.701, and 0.674, respectively). GRP78 showed high sensitivity and specificity as a biomarker in the diagnosis of sepsis. The AUC of CHOP for the diagnosis of sepsis was 0.813 (95% CI: 0.721–0.906). CHOP, as a biomarker, showed high specificity but poor sensitivity in the diagnosis of sepsis. The combination of GRP78 and CHOP was used for the diagnosis of sepsis, the maximum AUC was 0.852(95% CI: 0.765–0.939) and the optimal threshold was 0.57 (sensitivity, 84.6%; specificity, 73.1%).
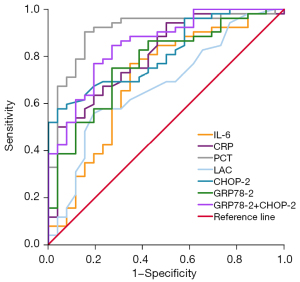
Table 4
| Number of days in the ICU | AUC | P values | Optimum threshold | Sensitivity (%) | Specific degrees (%) | Youden index |
|---|---|---|---|---|---|---|
| PCT | 0.910 | <0.001 | 2.6 | 88.5 | 84.6 | 0.731 |
| IL-6 | 0.701 | 0.004 | 46.3 | 76.9 | 65.4 | 0.423 |
| CRP | 0.802 | <0.001 | 111.31 | 63.5 | 80.8 | 0.443 |
| LAC | 0.674 | 0.013 | 1.75 | 55.8 | 80.8 | 0.366 |
| GRP78 d2 | 0.771 | <0.001 | 157.29 | 75.0 | 73.1 | 0.481 |
| CHOP d2 | 0.813 | <0.001 | 4.915 | 57.7 | 96.2 | 0.539 |
| GRP78 d2 + CHOP d2 | 0.852 | <0.001 | 0.57 | 84.6 | 73.1 | 0.577 |
AUC, area under the curve; ICU, intensive care unit; PCT, procalcitonin; IL, interleukin; CRP, C-reactive protein; LAC, lactic acid; GRP78, glucose regulated protein 78; CHOP, C/EBP homologous protein; d2, day 2 after admission.
Discussion
The traditional indicators commonly used in the clinical assessment of infections, such as PCT, CRP, IL-6, and LAC, can be influenced by many factors, especially in the elderly who are often complicated by low immune function and body response. Thus, elevated levels of these indicators may not accurately reflect the severity of disease. Studies using sepsis models have increasing demonstrated that CHOP and GRP78 are important mediators and markers of ERS (6,8-12). During the occurrence of sepsis, the ERS pathway is activated, and the expression of CHOP and GRP78 are enhanced. In this study, the serum levels of GRP78 and CHOP in the sepsis group were significantly higher than those in the non-sepsis infected group (P<0.05).
GRP78 is a signature molecular chaperone of ERS and a member of the heat shock protein 70 family (13). Under physiological action, GRP78 participates in the correct folding of proteins in the endoplasmic reticulum and assists in the transmembrane transport of proteins. GRP78 is an important molecule that promotes the normal synthesis and maturation of proteins and maintains the normal physiological function of cells (14). GRP78 binds to the transmembrane proteins the inositol-requiring enzyme 1α (IRE1α), PKR-like endoplasmic reticulum kinase (PERK), and transcription factor 6 (ATF6), and inhibits the activity of these transmembrane proteins, leaving them in an inactivated state (15,16). Under acute stress, GRP78 separates from the transmembrane proteins, activating the unfolded protein response (UPR). The UPR further promotes the expression of GRP78 chaperone proteins, and thus plays a protective role in the endoplasmic reticulum itself. Therefore, the upregulation of GRP78 expression is specific for the ERS response, and is considered to be a sensitive marker of ERS (17). Studies by Teng et al. (18) and Zhang et al. (8) demonstrated that GRP78 expression was elevated during lipopolysaccharide (LPS)-induced acute kidney injury, and that downregulation of GRP78 inhibited ERS, thereby reducing sepsis-associated acute kidney injury. In this current study, the serum GRP78 content of patients with sepsis was higher than that of non-septic patients and healthy controls. When GRP78 was used for the diagnosis of sepsis, the diagnostic efficiency was the highest on the second day, and the optimal threshold was 157.29 ng/L. In addition, it has good sensitivity and specificity in the diagnosis of sepsis, and can be used as one of the indicators for critically ill patients. Therefore, changes in GRP78 expression may be helpful for evaluating the severity of sepsis and guiding treatment regimens. However, this study found that GRP78 was not an effective prognostic predictor of sepsis.
CHOP is a specific transcription factor that mediates ERS-induced apoptosis. It is lowly expressed in normal cells, but is activated by IRE1α, PERK, and ATF6 during ERS, resulting in significantly increased mRNA and protein expression levels (19). A large number of studies (20-23) have shown that overexpression of CHOP can block the cell division cycle, negatively regulate cell growth, and induce cell apoptosis. Therefore, CHOP is considered to be the most important specific proapoptotic factor of ERS. Other investigations have also reported that the expression of CHOP is increased in sepsis mouse models, and inhibition of CHOP expression can reduce the systemic inflammatory response (8,23). Liu et al. also demonstrated upregulation of CHOP in rat sepsis models, and inhibition of the CHOP apoptotic signal alleviated sepsis-induced lung injury (9). In this study, the serum CHOP content of patients in the sepsis group was higher than that of patients in the non-sepsis infection group. When CHOP was used for the diagnosis of sepsis, the diagnostic efficacy was the highest on the second day, with an optimal threshold of 4.915 ng/L. While specificity was high, sensitivity was poor. These results suggested that CHOP may provide a novel direction for the accurate diagnosis of sepsis.
The poor homogeneity patients with sepsis, such as nutritional status, underlying diseases, and the presence of complications, may affect prognosis, and these factors should be investigated in future studies. Furthermore, the limited sample size of this study requires future studies with a larger sample size. GRP78 and CHOP may play a certain role or have a certain predictive value in the prognosis of patients with sepsis, however, more clinical trials are warranted to verify this. In conclusion, GRP78 and CHOP were effective as diagnostic markers for patients with sepsis.
Acknowledgments
Funding: The study was supported by the Scientific Research Project of Shanghai Municipal Commission of Health and Family Planning (No. 20174Y0126).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1445/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1445/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1445/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This research project was approved by the Ethics Committee of Shanghai East Hospital and Shanghai Cancer Center, Fudan University, and was implemented with the informed consent of the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801-10. [Crossref] [PubMed]
- Rizzo AN, Dudek SM. Endothelial Glycocalyx Repair: Building a Wall to Protect the Lung during Sepsis. Am J Respir Cell Mol Biol 2017;56:687-8. [Crossref] [PubMed]
- Weng L, Zeng XY, Yin P, et al. Sepsis-related mortality in China: a descriptive analysis. Intensive Care Med 2018;44:1071-80. [Crossref] [PubMed]
- Kibe S, Adams K, Barlow G. Diagnostic and prognostic biomarkers of sepsis in critical care. J Antimicrob Chemother 2011;66:ii33-40. [Crossref] [PubMed]
- Wacker C, Prkno A, Brunkhorst FM, et al. Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta-analysis. Lancet Infect Dis 2013;13:426-35. [Crossref] [PubMed]
- Ma S, Shao L, Liu Y, et al. A study of lymphocyte apoptosis and endoplasmic reticulum stress in the development of sepsis and their association with outcome in septic patients. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2015;27:115-20. [PubMed]
- Hou Y, Wang XF, Lang ZQ, et al. Adiponectin is protective against endoplasmic reticulum stress-induced apoptosis of endothelial cells in sepsis. Braz J Med Biol Res 2018;51:e7747. [Crossref] [PubMed]
- Zhang H, Zhang W, Jiao F, et al. The Nephroprotective Effect of MS-275 on Lipopolysaccharide (LPS)-Induced Acute Kidney Injury by Inhibiting Reactive Oxygen Species (ROS)-Oxidative Stress and Endoplasmic Reticulum Stress. Med Sci Monit 2018;24:2620-30. [Crossref] [PubMed]
- Liu W, Liu K, Zhang S, et al. Tetramethylpyrazine Showed Therapeutic Effects on Sepsis-Induced Acute Lung Injury in Rats by Inhibiting Endoplasmic Reticulum Stress Protein Kinase RNA-Like Endoplasmic Reticulum Kinase (PERK) Signaling-Induced Apoptosis of Pulmonary Microvascular Endothelial Cells. Med Sci Monit 2018;24:1225-31. [Crossref] [PubMed]
- Chen X, Wang Y, Xie X, et al. Heme Oxygenase-1 Reduces Sepsis-Induced Endoplasmic Reticulum Stress and Acute Lung Injury. Mediators Inflamm 2018;2018:9413876. [Crossref] [PubMed]
- Xu X, Liu Q, He S, et al. Qiang-Xin 1 Formula Prevents Sepsis-Induced Apoptosis in Murine Cardiomyocytes by Suppressing Endoplasmic Reticulum- and Mitochondria-Associated Pathways. Front Pharmacol 2018;9:818. [Crossref] [PubMed]
- Zhang D, Armstrong JS. Bax and the mitochondrial permeability transition cooperate in the release of cytochrome c during endoplasmic reticulum-stress-induced apoptosis. Cell Death Differ 2007;14:703-15. [Crossref] [PubMed]
- Zhang B, Liu Y, Zhang JS, et al. Cortistatin protects myocardium from endoplasmic reticulum stress induced apoptosis during sepsis. Mol Cell Endocrinol 2015;406:40-8. [Crossref] [PubMed]
- Boulangé-Lecomte C, Forget-Leray J, Xuereb B. Sexual dimorphism in Grp78 and Hsp90A heat shock protein expression in the estuarine copepod Eurytemora affinis. Cell Stress Chaperones 2014;19:591-7. [Crossref] [PubMed]
- Thon M, Hosoi T, Yoshii M, et al. Leptin induced GRP78 expression through the PI3K-mTOR pathway in neuronal cells. Sci Rep 2014;4:7096. [Crossref] [PubMed]
- Hong J, Kim K, Kim JH, et al. The Role of Endoplasmic Reticulum Stress in Cardiovascular Disease and Exercise. Int J Vasc Med 2017;2017:2049217. [Crossref] [PubMed]
- Stan RC, Silva RL, de Camargo MM. Corrigendum to "Human GRP78 affinity towards its signaling partners Ire1α and PERK is differently modulated by an unfolded protein client" [Biochem. Biophys. Res. Commun. 487 (2) (2017) 375-380]. Biochem Biophys Res Commun 2017;490:69. Erratum for Biochem Biophys Res Commun 2017;487:375-80. [Crossref] [PubMed]
- Teng J, Liu M, Su Y, et al. Down-regulation of GRP78 alleviates lipopolysaccharide-induced acute kidney injury. Int Urol Nephrol 2018;50:2099-107. [Crossref] [PubMed]
- Yi S, Shi W, Wang H, et al. Endoplasmic Reticulum Stress PERK-ATF4-CHOP Pathway Is Associated with Hypothalamic Neuronal Injury in Different Durations of Stress in Rats. Front Neurosci 2017;11:152. [Crossref] [PubMed]
- Bengesser SA, Fuchs R, Lackner N, et al. Endoplasmic Reticulum Stress and Bipolar Disorder - Almost Forgotten Therapeutic Drug Targets in the Unfolded Protein Response Pathway Revisited. CNS Neurol Disord Drug Targets 2016;15:403-13. [Crossref] [PubMed]
- van der Sanden MH, Houweling M, van Golde LM, et al. Inhibition of phosphatidylcholine synthesis induces expression of the endoplasmic reticulum stress and apoptosis-related protein CCAAT/enhancer-binding protein-homologous protein (CHOP/GADD153). Biochem J 2003;369:643-50. [Crossref] [PubMed]
- Doerflinger M, Reljic B, Menassa J, et al. Circulating BiP/Grp78 is a novel prognostic marker for sepsis-mediated immune cell death. FEBS J 2021;288:1809-21. [Crossref] [PubMed]
- Sun J, Wei S, Zhang Y, et al. Protective Effects of Astragalus Polysaccharide on Sepsis-Induced Acute Kidney Injury. Anal Cell Pathol (Amst) 2021;2021:7178253. [Crossref] [PubMed]
(English Language Editor: J. Teoh)


