
The value of WNT5A as prognostic and immunological biomarker in pan-cancer
Introduction
Cancer is a widespread disease and is the leading cause of death worldwide (1). Despite the rapid development of various treatment approaches for cancers in recent years, prognosis, especially in advanced cancers, remains poor (2,3). Excitingly, the advent of immunotherapy has revolutionized the clinical practice of oncology. At present, the expression level of PD-L1 in tumor cells and tumor mutational burden (TMB) are commonly used as biomarkers. However, how to successfully identify patients benefitting from immunotherapy is still the major challenge for clinicians. Hence, seeking novel targets and prognostic biomarkers, especially those related to immunotherapy, is of profound significance. With the improvement of R package (https://www.r-project.org/; The R Foundation for Statistical Computing, Vienna, Austria) and public databases such as The Cancer Genome Atlas (TCGA), more and more therapeutic targets of cancer are being discovered by performing pan-cancer expression analysis through bioinformatic analysis (4).
The WNT proteins are a large family of secreted glycoprotein signaling molecules rich in cysteine which play an important role in tumor progression, including proliferation, differentiation, apoptosis, and migration (5). At least 19 members of the WNT family have been identified and divided into 2 types: classical WNT/β-catenin signal molecules and non-classical signal molecules, according to their different biological functions (5,6). The WNT5A gene belongs to non-classical signaling molecules binding to different receptor complexes, and although its role in tumorigenesis is generally considered to be carcinogenic activities, controversy exists regarding its specific role (7). Several studies have reported that WNT5A has carcinogenic effects in lung cancer (8), gastric cancer (9), breast cancer (10), melanoma (11), and pancreatic cancer (12). But it has shown tumor suppressive effects in colon cancer (13), neuroblastoma (14), and thyroid cancer (15). Furthermore, conflicting effects have been recorded in the same tumor type. For example, Wu et al. found that WNT5A was highly expressed and has a carcinogenic effect in invasive esophageal squamous cell carcinoma (ESCC) (16). However, Li et al. reported that WNT5A is often silenced by promoter methylation and shows tumor inhibition characteristics in ESCC (17). Therefore, the role of WNT5A in cancer needs to be further elucidated and systematic bioinformatics analysis of WNT5A in pan-cancer is the preferred option.
To date, immune checkpoint blockade therapy has altered the treatment scheme of various tumors (18). However, the low response rate in some tumor types is mainly due to the highly immunosuppressive microenvironment and the absence of T cell infiltration, which is an urgent problem to be solved in immunotherapy (19). In addition, accumulating evidence has revealed a novel role of WNT5A in immunomodulation. The evidence suggests that WNT5A has a double effect on the tumor microenvironment. On one side, it can activate the ROR1/Akt/p65 pathway to promote inflammation and chemotaxis of immune cells (19,20); on the other side, it can activate TLR/MyD88/p50 to promote the synthesis of the anti-inflammatory cytokine interleukin 10 (IL-10) and immune tolerance (19,21). More importantly, inhibition of WNT5A signaling has been shown to increase the expression of programmed death-ligand 1 (PD-L1) in tumor tissues, and enhance the activity of anti-programmed cell death protein 1 (PD-1) and anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) antibodies, improving the response to checkpoint inhibitor therapy (22,23). For these reasons, it is of great significance to provide insight into the relationship of WNT5A and tumor immunity. We present the following article in accordance with the REMARK reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1317/rc).
Methods
In this study, we revealed the expression of WNT5A and its potential prognostic value in pan-cancer using TCGA, Genotype-Tissue Expression (GTEx), and Cancer Cell Line Encyclopedia (CCLE) datasets. We then performed correlation analysis between WNT5A expression level and immune checkpoints, tumor-infiltrating immune cells, TMB, and microsatellite instability (MSI), which are closely related to immunotherapy. Finally, we performed gene set enrichment analysis (GSEA) to identify the signaling pathways linked to WNT5A. Taken together, our pan-cancer analyses provide insights into the prognostic and immunotherapy role of WNT5A in various cancers.
Data acquisition
We downloaded WNT5A expression data of tumor and normal samples coupled with clinical information from TCGA (https://portal.gdc.cancer.gov) and GTEx dataset (https://commonfund.nih.gov/GTEx/). The WNT5A expression data of tumor cell lines were obtained from CCLE dataset (https://portals.broadinstitute.org/ccle). Moreover, cancer immune infiltration scores were analyzed with data from the Tumor Immune Estimation Resource (TIMER) database. The R package was used to analyze the data. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The full name and abbreviation of all the tumors are listed in Table 1.
Table 1
| Abbreviations | Tumor name |
|---|---|
| ACC | Adrenocortical carcinoma |
| BLCA | Bladder cancer |
| BRCA | Breast cancer |
| CESC | Cervical squamous cell carcinoma and endocervical adenocarcinoma |
| CHOL | Cholangiocarcinoma |
| COAD | Colon adenocarcinoma |
| COADREAD | Colon and rectal cancer |
| DLBC | Lymphoid neoplasm diffuse large B-cell lymphoma |
| ESCA | Esophageal carcinoma |
| GBM | Glioblastoma multiforme |
| GBMLGG | Glioblastoma multiforme low-grade glioma |
| HNSC | Head and neck squamous cell carcinoma |
| KICH | Kidney chromophobe |
| KIPAN | Pan-kidney cohort (KICH+KIRC+KIRP) |
| KIRC | Kidney renal clear cell carcinoma |
| KIRP | Kidney renal papillary cell carcinoma |
| LAML | Acute myeloid leukemia |
| LGG | Lower grade glioma |
| LIHC | Liver hepatocellular carcinoma |
| LUAD | Lung adenocarcinoma |
| LUSC | Lung squamous cell carcinoma |
| MESO | Mesothelioma |
| OV | Ovarian cancer |
| PAAD | Pancreatic adenocarcinoma |
| PCPG | Pheochromocytoma and paraganglioma |
| PRAD | Prostate adenocarcinoma |
| READ | Rectum adenocarcinoma |
| SARC | Sarcoma |
| STAD | Stomach adenocarcinoma |
| SKCM | Skin cutaneous melanoma |
| STES | Stomach and esophageal carcinoma |
| TGCT | Testicular germ cell tumors |
| THCA | Thyroid carcinoma |
| THYM | Thymoma |
| UCEC | Uterine corpus endometrial carcinoma |
| UCS | Uterine carcinosarcoma |
| UVM | Uveal melanoma |
| OS | Osteosarcoma |
| ALL | Acute lymphoblastic leukemia |
| NB | Neuroblastoma |
| WT | High-risk Wilms tumor |
Analysis of WNT5A expression levels
Kruskal-Wallis test line analysis of the WNT5A expression data was conducted to compare WNT5A messenger RNA (mRNA) expression in 31 different normal tissues and 21 various cancer cell lines. Then, the WNT5A expression levels compared between cancer and normal samples were evaluated with data solely from TCGA database. In addition, considering the small size of non-cancerous tissues in TCGA, the WNT5A expression data of the GTEx and TCGA databases was further analyzed.
Correlation analysis of WNT5A expression level and prognosis in pan-cancer
Survival analysis of the expression and survival data obtained from TCGA in pan-cancer was conducted to confirm the prognostic role of WNT5A in pan-cancer. For the predictive analysis, a one-way Cox regression test was used to reveal the correlation between WNT5A expression and patient survival. Furthermore, the Kaplan-Meier (K-M) test was used to analyze patient survival. Prognostic indicators consisted of overall survival (OS), disease-specific survival (DSS), disease-free interval (DFI), and progression-free interval (PFI). The results were presented in the form of forest plots (Cox regression test) and survival curves (K-M test).
Correlation analysis of the role of WNT5A in immune infiltration and tumor microenvironment
To evaluate the performance of WNT5A in immune infiltration, Spearman’s rank correlation coefficient was utilized to distinguish the role of WNT5A in immune cell infiltration, including B cells, CD4+ T cells, CD8+ T cells, macrophages, neutrophils, and dendritic cells (DCs). Furthermore, we implemented an Estimation of Stromal and Immune Cells in Malignant Tumors Using Expression data (ESTIMATE) algorithm to assess the tumor microenvironment-related scores obtained from the above mentioned databases.
Correlation analysis of WNT5A expression level and immune checkpoints and neoantigens
To further clarify the correlation between WNT5A and tumor immune activity, immune checkpoints and neoantigens were analyzed. Spearman’s rank correlation coefficient was performed to analyze the relationship between the expression of WNT5A and immune checkpoints, which were segregated into inhibitory and stimulatory groups. In addition, the number of neoantigens in every sample was detected and counted using a scanner, and the analysis mentioned above was applied to evaluate the correlation of WNT5A expression and the neoantigens number.
Correlation analysis of WNT5A expression level and TMB and MSI
The TMB is a quantifiable biomarker reflecting the mutational number of a tumor cell; MSI refers to the occurrence of a new microsatellite allele phenomenon compared with normal tissue (24). Correlation of WNT5A expression with TMB and MSI was analyzed utilizing Pearson’s correlation coefficient. Bubble charts were used to present the results.
GSEA
It is common for GSEA to be utilized to analyze and explain changes in the level of coordination pathways (25). The signaling pathway of WNT5A was analyzed by GSEA analysis with the R package clusterProfiler. The Kyoto Encyclopedia of Genes and Genomes (KEGG database; KEGG; https://www.kegg.jp.) and hallmark gene sets from the Molecular Signature Database (MsigDB) were applied. Pathways with normalized enrichment score |NES| >1.5, false discovery rate (FDR) <0.25, and P<0.01 were considered significantly enriched.
Statistical analysis
Statistical analysis methods were described in the above parts. A value of P<0.05 (two-side) was considered significant.
Results
WNT5A is highly expressed in most cancers
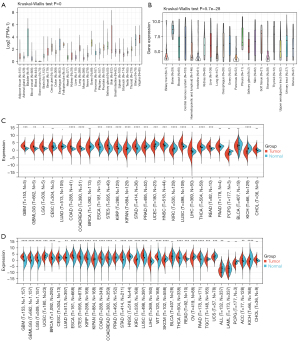
Data from the CCLE database, GTEx dataset, and TCGA database were analyzed to evaluate the WNT5A expression in normal and tumor tissues. Data from the GTEx dataset showed WNT5A was normally expressed in 31 normal tissues, with higher expression levels present in the bladder, uterus, and vagina, and lower expression levels in blood and bone marrow (Figure 1A). The CCLE analysis demonstrated that WNT5A is more highly expressed in bone, soft tissue, and the thyroid, while more lowly expressed in biliary tract, intestine, pancreas, and stomach (Figure 1B). In order to explore the expression level of WNT5A in tumor and matched normal tissues, we first analyzed the data from TCGA database separately (Figure 1C), and then analyzed the data from both TCGA and GTEx datasets. These results showed that WNT5A expression was elevated (19/34, 55.88%) in lung squamous cell carcinoma (LUSC), lung adenocarcinoma (LUAD), esophageal carcinoma (ESCA), glioblastoma multiforme (GBM), glioblastoma multiforme low-grade glioma (GBMLGG), low-grade glioma (LGG), breast cancer (BRCA), stomach and esophageal carcinoma (STES), kidney renal papillary cell carcinoma (KIRP), colon adenocarcinoma (COAD), colon and rectal cancer (COADREAD), stomach adenocarcinoma (STAD), head and neck squamous cell carcinoma (HNSC), liver hepatocellular carcinoma (LIHC), high-risk wilms tumor (WT), thyroid cancer (THCA), rectum adenocarcinoma (READ), pancreatic adenocarcinoma (PAAD), and adrenocortical carcinoma (ACC). However, WNT5A expression was lowered (10/34, 29.41%) in uterine corpus endometrial carcinoma (UCEC), KIPAN, prostate adenocarcinoma (PRAD), kidney renal clear cell carcinoma (KIRC), Skin cutaneous melanoma (SKCM), ovarian cancer (OV), testicular germ cell tumors (TGCT), uterine carcinosarcoma (UCS), acute lymphoblastic leukemia (ALL), and kidney chromophobe (KICH) (Figure 1D). These results revealed that the expression level of WNT5A is generally higher in the majority of tumors than that in corresponding normal tissues.
WNT5A is associated with prognosis in pan-cancer
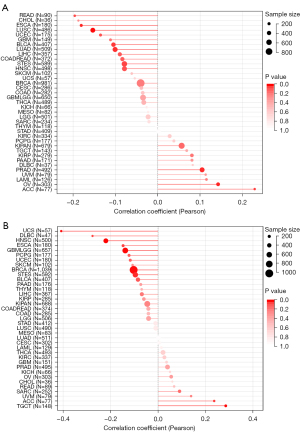
To study the association between WNT5A expression and prognosis, we performed a survival association analysis for each cancer, including OS, DSS, DFI, and PFI. Cox proportional hazards model analysis showed that WNT5A expression levels were associated with OS in GBMLGG (HR =1.40, P<0.01), LGG (HR =1.39, P<0.01), ACC (HR =1.45, P<0.01), PAAD (HR =1.27, P<0.01), KIRC (HR =1.16, P=0.02), COADREAD (HR =0.86, P=0.02), and READ (HR =0.70, P=0.02) (Figure 2A). The K-M survival analysis revealed high expression of WNT5A was associated with poor OS in LGG (P<0.01, Figure 2B), ACC (P<0.01, Figure 2C), PAAD (P<0.01, Figure 2D), and KIRC (P<0.01, Figure 2E). In addition, Cox analysis results also revealed that WNT5A expression levels were associated with DSS in GBMLGG (HR =1.46, P<0.01), LGG (HR =1.44, P<0.01), ACC (HR =1.52, P<0.01), PAAD (HR =1.30, P<0.01), KIRP (HR =0.75, P<0.01), and LUSC (HR =0.86, P=0.01) (Figure 3A). Similarly, K-M survival analysis revealed that high expression of WNT5A was associated with poor DSS in LGG (P<0.01, Figure 3B), ACC (P<0.01, Figure 3C), PAAD (P<0.01, Figure 3D), and KIRC (P<0.01, Figure 3E), while higher expression level of WNT5A was associated with better DSS in KIRP (P<0.01, Figure 3F).

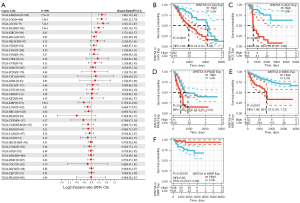
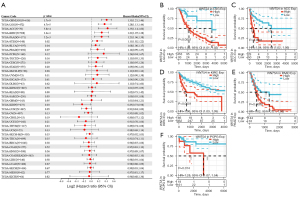
Moreover, regarding associations between WNT5A expression and DFI, Cox analysis depicted the relationship in PAAD (HR =2.49, P<0.01), COAD (HR =0.59, P<0.01), BRCA (HR =0.88, P=0.04), and COADREAD (HR =0.69, P=0.04) (Figure 4A). The K-M survival analysis revealed that high expression of WNT5A was associated with poor DFI in ESCA (P=0.021, Figure 4B), HNSC (P=0.03, Figure 4C), and PAAD (P<0.01, Figure 4D). Furthermore, Cox analysis found WNT5A expression was associated with PFI in GBMLGG (HR =1.33, P<0.01), LGG (HR =1.28, P<0.01), ACC (HR =1.35, P<0.01), KIRC (HR =1.21, P<0.01), PAAD (HR =1.21, P<0.01), STES (HR =1.11, P=0.02), bladder cancer (BLCA; HR =1.08, P=0.04), and KIRP (HR =0.85, P=0.03) (Figure 5A). The K-M survival analysis showed that high expression of WNT5A was associated with poor PFI in LGG (P<0.01, Figure 5B), ACC (P<0.01, Figure 5C), KIRC (P<0.01, Figure 5D), PAAD (P<0.01, Figure 5E), and PCPG (P=0.014, Figure 5F).

WNT5A affects tumor immune infiltration and microenvironment in pan-cancer
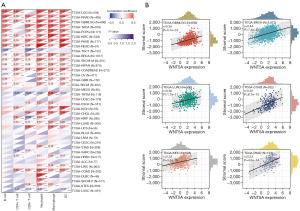
Tumor immune infiltration refers to the transfer of immune cells from blood to tumor tissues (26). To explore the role of WNT5A in tumor immunity, we first performed correlation analysis of WNT5A expression and various immune cells. Our data revealed the positive correlations between them in most cancers, especially in READ, PAAD, KIRC, LGG, PRAD, GBMLGG, THCA, PCPG, BRCA, COADREAD, and COAD, while negative correlations in TGCT and LUSC. But in thymoma (THYM), positive correlation with macrophages and negative correlation with CD4+ T cells, CD8+ T cells, neutrophils, and DCs were found simultaneously. Furthermore, among the data of immune cells, WNT5A expression was found to be positively associated with neutrophils and macrophages in 26 tumors, and DCs in 22 tumors (Figure 6A). In order to explore the effect of WNT5A expression on tumor microenvironment, we used the ESTIMATE algorithm to evaluate the correlation between WNT5A expression and stromal score. Results revealed the WNT5A expression was positively correlated with the stromal score in LUAD, GBMLGG, BRCA, COAD, KIRC, and PAAD (Figure 6B). In conclusion, these results demonstrate that WNT5A may promote immune cell infiltration in the tumor microenvironment (TME).
WNT5A is correlated with immune checkpoints and immune neoantigens in pan-cancer
The data presented above highlight a potential role for WNT5A in tumor immunity. Based on these findings, we performed correlation analysis of WNT5A expression and immune checkpoints, which included 24 immune inhibitors and 36 stimulators. Among the data of immune inhibitors in the 40 tumors, we found that WNT5A expression was positively linked to VEGFA in 23 tumors; to CD274 (PD-L1) in 20 tumors; to IL10 in 29 tumors; to CD276 in 34 tumors; to EDNRB in 29 tumors; to CTLA4 in 21 tumors; to IL12A in 22 tumors; to VTCN1 in 25 tumors; to TGFB1 in 28 tumors; to HAVCR2 in 26 tumors; to C10orf54 in 27 tumors; and to BTLA in 23 tumors. Additionally, among the data of immune stimulators, WNT5A expression was found to be positively associated with CX3CL1 in 23 tumors; HMGB1 in 28 tumors; ENTPD1 in 30 tumors; TLR4 in 32 tumors; tumor necrosis factor (TNF) SF4 in 33 tumors; BTN3A in 29 tumors; BTN3A2 in 23 tumors; CD40 in 25 tumors; ICAM1 in 26 tumors; IL1A in 29 tumors; IL1B in 27 tumors; TNF in 25 tumors; TNFRSF9 in 24 tumors; CD80 in 27 tumors; IL2RA in 28 tumors; ITGB2 in 23 tumors; CD28 in 27 tumors; and CD40LG in 24 tumors. Moreover, WNT5A expression was positively associated with 19 of 24 immune inhibitors and 29 of 36 immune stimulators in COADERAD; 17 of 24 immune inhibitors and 33 of 36 immune stimulators in neuroblastoma (NB); 18 of 24 immune inhibitors and 32 of 36 immune stimulators in PAAD; 17 of 24 immune inhibitors and 30 of 36 immune stimulators in uveal melanoma (UVM); 18 of 24 immune inhibitors and 28 of 36 immune stimulators in OV; 21 of 24 immune inhibitors and 30 of 36 immune stimulators in PRAD; 17 of 24 immune inhibitors and 31 of 36 immune stimulators in GBMLGG; and 18 of 24 immune inhibitors and 29 of 36 immune stimulators in LGG. Conversely, WNT5A expression was negatively associated with 10 of 24 immune inhibitors and 19 of 36 immune stimulators in LUSC, and 11 of 24 immune inhibitors and 17 of 36 immune stimulators in TGCT (Figure 7A). Next, results of neoantigens analysis suggested that WNT5A expression was negatively associated with the number of neoantigens in LUAD, LUSC, BRCA, UCEC, and SKCM, while positively associated with KIRP and HNSC (Figure 7B).
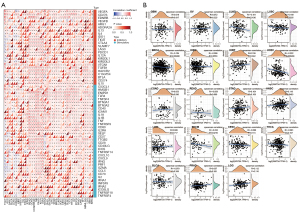
WNT5A is associated with TMB and MSI
Tumors are diseases caused by genetic mutations, while TMB and MSI can reflect the change of genomic instability (27). We found that WNT5A expression was positively correlated to TMB in ACC and OV, but negatively correlated to it in LUSC, ESCA, and READ (Figure 8A). Similarly, WNT5A expression was found to be positively associated with MSI in TGCT and ACC, but negatively associated with it in UCS, lymphoid neoplasm diffuse large B-cell lymphoma (DLBC), and HNSC (Figure 8B).
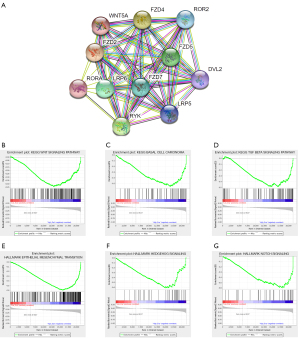
WNT5A is implicated in the regulation of numerous signaling pathways
In order to clarify the relevant mechanisms, we firstly performed protein-protein interaction (PPI) network analysis to reveal the functional network of WNT5A. The results showed that WNT5A was linked to FZD2, FZD4, FZD5, FZD7, LRP5, LRP6, ROR2, RORA, DVL2, and RYK, most of which have been demonstrated to be related to the WNT signaling pathway (Figure 9A). Then GSEA was used to analyze the data of high and low expression groups of WNT5A. The results indicated that the KEGG WNT signaling pathway (Figure 9B), KEGG basal cell carcinoma (Figure 9C), KEGG TGFβ signaling pathway (Figure 9D), hallmark epithelial-mesenchymal transition (EMT; Figure 9E), hallmark Hedgehog signaling (Figure 9F), and hallmark Notch signaling (Figure 9G) was highly enriched in the WNT5A high expression group.
Discussion
With the widespread use of immunotherapy and targeted therapy, the prognosis of tumor patients has improved (1). However, due to the heterogeneity of various patients, the OS of cancer patients remains poor (1,28). For this reason, the search for new therapeutic targets related to immunotherapy has received increasing attention from researchers. From another aspect, a pan-cancer analysis can provide broad insights about the role of a gene from many aspects in various cancers through mining major databases, which is an effective method to search for intriguing targets for tumor therapy (3).
As a non-classical WNT signal molecule, WNT5A is highly conserved between species and plays a key role in embryonic development, pathological disorders, and internal environmental balance (29). Due to its important role in embryonic development, the expression level of WNT5A is high in various organs and tissues during the embryonic stage, but generally decreased in adult tissues (7,30,31). It has been demonstrated that WNT5A expression increases when immune cells are exposed to pathogens (32). Interestingly, our data from the GTEx dataset showed WNT5A was more highly expressed in the bladder, uterus, and vagina. As we know, these cavities, which are often exposed to bacteria, are prone to various forms of inflammation and immune cell congregation. Therefore, we can speculate that the high expression of WNT5A may be related to inflammatory cell infiltration and immune cells aggregation.
As a potential prognostic marker of cancer, WNT5A has anticancer or oncogenic activity, depending on tumor type and stages, and can regulate TME, inflammation, proliferation, EMT, and metabolism in cancer (7,33). Our analysis of the TCGA and GTEx datasets in 34 common tumors revealed that WNT5A expression was elevated in 19 tumors and lowered in 10 tumors, suggesting that it is overexpressed in most tumors but low expressed in some tumors. Among the positive results, the overexpression of WNT5A in some tumor species has been reported, such as LUSC (8), LUAD (8), ESCA (16), GBM (34), BRCA (35), PAAD (12), and ACC (36), while some of them have not been reported, such as GBMLGG, LGG, HNSC, and so on. Furthermore, combining previous literature with our survival analysis of OS, DSS, DFI, and PFI, among the WNT5A overexpression tumors, we found that WNT5A expression was associated with poor prognosis in ESCA (16), LGG, ACC (36), PAAD (12), and HNSC. Conversely, high WNT5A expression was correlated to longer DSS in KIRP, which warrants further research.
By analyzing recent studies on WNT5A and tumor immunity, Lopez-Bergami and Barbero proposed that WNT5A overproduced by the tumor cell could foster a pro-inflammatory milieu and induce immune cells chemotaxis. When immune cells were recruited, WNT5A induced a tolerogenic phenotype of mononuclear phagocytes in myelomonocytic cells via the TLR/MyD88/P50 pathway (19,37). Our correlation analysis revealed that in most cancers, WNT5A expression was positively correlated with various immune cells, especially neutrophils, macrophages, and DCs. Otherwise, the correlation analysis using ESTIMATE algorithm showed that WNT5A expression was positively correlated with the stromal score in LUAD, GBMLGG, BRCA, COAD, KIRC, and PAAD. Those results showed that WNT5A expression may be associated with promoting inflammation, but as neutrophils, macrophages, and DCs also play an important role in immune suppression (23,38); the role of WNT5A in promoting immune tolerance also needs to be noted.
To date, many immune checkpoints have been identified and studied, and WNT5A expression is thought to stimulate a variety of cytokines, including immune stimulators and inhibitors, which in turn cause inflammation or further stimulate immune tolerance (19,39). Our data revealed that WNT5A expression was positively linked to multiple immune inhibitors, such as VEGFA, PD-L1 (CD274), IL10, CD276, EDNRB, CTLA4, IL12A, and TGFB1, and various immune stimulators, such as HMGB1, ENTPD1, TLR4, TNFSF4, BTN3A, ICAM1, IL1A, IL1B, and TNF. Some of these cytokines have been reported to be associated with WNT5A, such as VEGFA (8), PD-L1 (22,37), IL10 (40), CTLA4 (23), IL1A (41), IL1B (41), and TNF (42). Furthermore, the results of neoantigen analysis suggested that WNT5A expression was associated with the number of neoantigens in LUAD, LUSC, BRCA, UCEC, SKCM, KIRP, and HNSC. Currently, PDL1 and CTLA4 are the main therapeutic targets of immunotherapy. Interestingly, WNT5A has been found to be associated with them, and inhibition of WNT5A can promote the effect of immunotherapy drugs (22,23), indicating that WNT5A may be a potential target of immunotherapy. In addition, we also found that the relationship between WNT5A expression and cytokines is not consistent in different tumors, suggesting the influence of tumor heterogeneity on WNT5A-targeted immunotherapy.
Both TMB and MSI tend to be predictive markers of immune checkpoint inhibitors (ICIs), which is important for identifying patients with potential for ICIs in various cancers (24,43). Our results revealed that WNT5A expression was positively correlated with TMB in ACC and OV, while negatively correlated to LUSC, ESCA, and READ. In addition, WNT5A expression was positively associated with MSI in TGCT and ACC, while negatively associated with MSI in UCS, DLBC, and HNSC. It is especially noteworthy that WNT5A expression was positively correlated with TMB and MSI in ACC. At present, the direct relationship between WNT5A and ACC has not been reported, but the carcinogenic effect of WNT/β-catenin in ACC has been revealed (44,45). Therefore, the relationship between WNT5A and tumor immunity in ACC warrants further confirmation.
The WNT5A gene could stimulate non-canonical WNT pathway as well as activate or antagonize the canonical WNT signaling pathway by binding to different receptors or co-receptor complexes, such as Frizzled (FZD), receptor tyrosine kinase-like orphan receptor-1 and 2 (ROR1/2), receptor related to tyrosine kinases (RYK), low-density lipoprotein receptor-related protein 5/6 (LRP5/6), and DVL, thus playing a crucial role in tumor development (29,46-48). Our PPI network analysis showed that WNT5A was linked to FZD2, FZD4, FZD5, FZD7, LRP5, LRP6, ROR2, DVL2, RYK, and RORA, most of which have been reported to be members of the WNT signaling pathway. Interestingly, there have been no direct studies on RORA and WNT5A until now, but it has been reported that RORA can encode the transcription activator RORα and further attenuates WNT/β-Catenin signaling in colon cancer (49,50), indicating the potential correlation between them, which needs to be further evaluated.
As an important molecule of the WNT signaling pathway, WNT5A can interact with TGFβ, Notch, or other pathways to regulate EMT and immunity in cancer (7,51,52). Our GSEA analysis also indicated that the KEGG WNT signaling pathway, KEGG basal cell carcinoma, KEGG TGFβ signaling pathway, hallmark epithelial-mesenchymal transition, hallmark Notch signaling, and hallmark Hedgehog signaling was highly enriched in the WNT5A high expression group. Previous studies have suggested the following functions: (I) WNT5A can regulate TGFβ1 to promote immunosuppression in melanoma (53); (II) in psoriasis, WNT5A and Notch1 signaling can influence each other and regulate the secretion of cytokines IL-12, IL-23, and TNF-α, which is related to immunity (54); (III) until now, no experimental reports on the interaction between WNT5A and Hedgehog signaling have been retrieved in the field of tumor immunity. However, it has been shown that Hedgehog signaling can mediate how WNT/β-catenin induces cartilage and bone tumor formation (55). Therefore, as a member of WNT family, the interaction between WNT5A and Hedgehog signaling in tumor immunity may be a feasible research direction.
In summary, we analyzed the expression and prognosis of WNT5A in different tumors, indicating that WNT5A is correlated with the prognosis of tumors. On this basis, we further revealed that WNT5A was associated with tumor immune, suggesting that it may be a potential immunological biomarker and therapeutic target in cancer. Of course, these conclusions were obtained by bioinformatical analyses of open accessible databases, for which there is a lack of experimental verification, but they still provide some evidence and have a certain significance for further research.
Acknowledgments
Funding: Our research was supported by the National Natural Science Foundation of China (Nos. 82173252, 81871866), the Shaanxi Social Development Science and Technology Key Project (Nos. 2016SF-308; 2019SF-033), Natural Science Foundation of Shaanxi Province (No. 2022JQ-862), and the Project of Tangdu Hospital, The Fourth Military Medical University (No. 2018 Key Talents).
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1317/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1317/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. Erratum in: CA Cancer J Clin 2021;71:359. [Crossref] [PubMed]
- Duan H, Wang T, Luo Z, et al. Neoadjuvant programmed cell death protein 1 inhibitors combined with chemotherapy in resectable non-small cell lung cancer: an open-label, multicenter, single-arm study. Transl Lung Cancer Res 2021;10:1020-8. [Crossref] [PubMed]
- Zhang Z, Zhang X, Huang A. Aggresome-Autophagy Associated Gene HDAC6 Is a Potential Biomarker in Pan-Cancer, Especially in Colon Adenocarcinoma. Front Oncol 2021;11:718589. [Crossref] [PubMed]
- Cheng X, Wang X, Nie K, et al. Systematic Pan-Cancer Analysis Identifies TREM2 as an Immunological and Prognostic Biomarker. Front Immunol 2021;12:646523. [Crossref] [PubMed]
- Parsons MJ, Tammela T, Dow LE. WNT as a Driver and Dependency in Cancer. Cancer Discov 2021;11:2413-29. [Crossref] [PubMed]
- Zeng G, Awan F, Otruba W, et al. Wnt'er in liver: expression of Wnt and frizzled genes in mouse. Hepatology 2007;45:195-204. [Crossref] [PubMed]
- Asem MS, Buechler S, Wates RB, et al. Wnt5a Signaling in Cancer. Cancers (Basel) 2016;8:79. [Crossref] [PubMed]
- Huang CL, Liu D, Nakano J, et al. Wnt5a expression is associated with the tumor proliferation and the stromal vascular endothelial growth factor--an expression in non-small-cell lung cancer. J Clin Oncol 2005;23:8765-73. [Crossref] [PubMed]
- Shojima K, Sato A, Hanaki H, et al. Wnt5a promotes cancer cell invasion and proliferation by receptor-mediated endocytosis-dependent and -independent mechanisms, respectively. Sci Rep 2015;5:8042. [Crossref] [PubMed]
- Hasan MK, Widhopf GF 2nd, Zhang S, et al. Wnt5a induces ROR1 to recruit cortactin to promote breast-cancer migration and metastasis. NPJ Breast Cancer 2019;5:35. [Crossref] [PubMed]
- Da Forno PD, Pringle JH, Hutchinson P, et al. WNT5A expression increases during melanoma progression and correlates with outcome. Clin Cancer Res 2008;14:5825-32. [Crossref] [PubMed]
- Bo H, Zhang S, Gao L, et al. Upregulation of Wnt5a promotes epithelial-to-mesenchymal transition and metastasis of pancreatic cancer cells. BMC Cancer 2013;13:496. [Crossref] [PubMed]
- Lund CM, Dyhl-Polk A, Nielsen DL, et al. Wnt5a expression and prognosis in stage II-III colon cancer. Transl Oncol 2021;14:100892. [Crossref] [PubMed]
- Blanc E, Roux GL, Bénard J, et al. Low expression of Wnt-5a gene is associated with high-risk neuroblastoma. Oncogene 2005;24:1277-83. [Crossref] [PubMed]
- Kremenevskaja N, von Wasielewski R, Rao AS, et al. Wnt-5a has tumor suppressor activity in thyroid carcinoma. Oncogene 2005;24:2144-54. [Crossref] [PubMed]
- Wu X, Yan T, Hao L, et al. Wnt5a induces ROR1 and ROR2 to activate RhoA in esophageal squamous cell carcinoma cells. Cancer Manag Res 2019;11:2803-15. [Crossref] [PubMed]
- Li J, Ying J, Fan Y, et al. WNT5A antagonizes WNT/β-catenin signaling and is frequently silenced by promoter CpG methylation in esophageal squamous cell carcinoma. Cancer Biol Ther 2010;10:617-24. [Crossref] [PubMed]
- He X, Xu C. Immune checkpoint signaling and cancer immunotherapy. Cell Res 2020;30:660-9. [Crossref] [PubMed]
- Lopez-Bergami P, Barbero G. The emerging role of Wnt5a in the promotion of a pro-inflammatory and immunosuppressive tumor microenvironment. Cancer Metastasis Rev 2020;39:933-52. [Crossref] [PubMed]
- Jung YS, Lee HY, Kim SD, et al. Wnt5a stimulates chemotactic migration and chemokine production in human neutrophils. Exp Mol Med 2013;45:e27. [Crossref] [PubMed]
- Cao S, Zhang X, Edwards JP, et al. NF-kappaB1 (p50) homodimers differentially regulate pro- and anti-inflammatory cytokines in macrophages. J Biol Chem 2006;281:26041-50. [Crossref] [PubMed]
- Zhao F, Xiao C, Evans KS, et al. Paracrine Wnt5a-β-Catenin Signaling Triggers a Metabolic Program that Drives Dendritic Cell Tolerization. Immunity 2018;48:147-160.e7. [Crossref] [PubMed]
- Holtzhausen A, Zhao F, Evans KS, et al. Melanoma-Derived Wnt5a Promotes Local Dendritic-Cell Expression of IDO and Immunotolerance: Opportunities for Pharmacologic Enhancement of Immunotherapy. Cancer Immunol Res 2015;3:1082-95. [Crossref] [PubMed]
- Schrock AB, Ouyang C, Sandhu J, et al. Tumor mutational burden is predictive of response to immune checkpoint inhibitors in MSI-high metastatic colorectal cancer. Ann Oncol 2019;30:1096-103. [Crossref] [PubMed]
- Powers RK, Goodspeed A, Pielke-Lombardo H, et al. GSEA-InContext: identifying novel and common patterns in expression experiments. Bioinformatics 2018;34:i555-64. [Crossref] [PubMed]
- Zhang B, Tang B, Gao J, et al. A hypoxia-related signature for clinically predicting diagnosis, prognosis and immune microenvironment of hepatocellular carcinoma patients. J Transl Med 2020;18:342. [Crossref] [PubMed]
- Yates LR, Campbell PJ. Evolution of the cancer genome. Nat Rev Genet 2012;13:795-806. [Crossref] [PubMed]
- Gavrielatou N, Doumas S, Economopoulou P, et al. Biomarkers for immunotherapy response in head and neck cancer. Cancer Treat Rev 2020;84:101977. [Crossref] [PubMed]
- Astudillo P. An emergent Wnt5a/YAP/TAZ regulatory circuit and its possible role in cancer. Semin Cell Dev Biol 2022;125:45-54. [Crossref] [PubMed]
- Kumawat K, Gosens R. WNT-5A: signaling and functions in health and disease. Cell Mol Life Sci 2016;73:567-87. [Crossref] [PubMed]
- Bakker ER, Raghoebir L, Franken PF, et al. Induced Wnt5a expression perturbs embryonic outgrowth and intestinal elongation, but is well-tolerated in adult mice. Dev Biol 2012;369:91-100. [Crossref] [PubMed]
- Nanbara H, Wara-aswapati N, Nagasawa T, et al. Modulation of Wnt5a expression by periodontopathic bacteria. PLoS One 2012;7:e34434. [Crossref] [PubMed]
- Astudillo P. Wnt5a Signaling in Gastric Cancer. Front Cell Dev Biol 2020;8:110. [Crossref] [PubMed]
- Yu JM, Jun ES, Jung JS, et al. Role of Wnt5a in the proliferation of human glioblastoma cells. Cancer Lett 2007;257:172-81. [Crossref] [PubMed]
- Lejeune S, Huguet EL, Hamby A, et al. Wnt5a cloning, expression, and up-regulation in human primary breast cancers. Clin Cancer Res 1995;1:215-22. [PubMed]
- Mermejo LM, Leal LF, Colli LM, et al. Altered expression of noncanonical Wnt pathway genes in paediatric and adult adrenocortical tumours. Clin Endocrinol (Oxf) 2014;81:503-10. [Crossref] [PubMed]
- Valencia J, Hernández-López C, Martínez VG, et al. Wnt5a skews dendritic cell differentiation to an unconventional phenotype with tolerogenic features. J Immunol 2011;187:4129-39. [Crossref] [PubMed]
- Mao Y, Poschke I, Kiessling R. Tumour-induced immune suppression: role of inflammatory mediators released by myelomonocytic cells. J Intern Med 2014;276:154-70. [Crossref] [PubMed]
- Bergenfelz C, Medrek C, Ekström E, et al. Wnt5a induces a tolerogenic phenotype of macrophages in sepsis and breast cancer patients. J Immunol 2012;188:5448-58. [Crossref] [PubMed]
- Liu Q, Yang C, Wang S, et al. Wnt5a-induced M2 polarization of tumor-associated macrophages via IL-10 promotes colorectal cancer progression. Cell Commun Signal 2020;18:51. [Crossref] [PubMed]
- Li S, Wang W, Zhang N, et al. IL-1β mediates MCP-1 induction by Wnt5a in gastric cancer cells. BMC Cancer 2014;14:480. [Crossref] [PubMed]
- Alquézar C, de la Encarnación A, Moreno F, et al. Progranulin deficiency induces overactivation of WNT5A expression via TNF-α/NF-κB pathway in peripheral cells from frontotemporal dementia-linked granulin mutation carriers. J Psychiatry Neurosci 2016;41:225-39. [Crossref] [PubMed]
- Litchfield K, Reading JL, Puttick C, et al. Meta-analysis of tumor- and T cell-intrinsic mechanisms of sensitization to checkpoint inhibition. Cell 2021;184:596-614.e14. [Crossref] [PubMed]
- Rubin B, Pilon C, Pezzani R, et al. The effects of mitotane and 1α,25-dihydroxyvitamin D on Wnt/beta-catenin signaling in human adrenocortical carcinoma cells. J Endocrinol Invest 2020;43:357-67. [Crossref] [PubMed]
- Borges KS, Pignatti E, Leng S, et al. Wnt/β-catenin activation cooperates with loss of p53 to cause adrenocortical carcinoma in mice. Oncogene 2020;39:5282-91. [Crossref] [PubMed]
- Prasad CP, Chaurasiya SK, Guilmain W, et al. WNT5A signaling impairs breast cancer cell migration and invasion via mechanisms independent of the epithelial-mesenchymal transition. J Exp Clin Cancer Res 2016;35:144. [Crossref] [PubMed]
- Yamaguchi TP, Bradley A, McMahon AP, et al. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development 1999;126:1211-23. [Crossref] [PubMed]
- Katoh M, Katoh M. WNT signaling pathway and stem cell signaling network. Clin Cancer Res 2007;13:4042-5. [Crossref] [PubMed]
- Salehi M, Kamali E, Karahmadi M, et al. RORA and Autism in The Isfahan Population: Is There An Epigenetic Relationship. Cell J 2017;18:540-6. [PubMed]
- Lee JM, Kim IS, Kim H, et al. RORalpha attenuates Wnt/beta-catenin signaling by PKCalpha-dependent phosphorylation in colon cancer. Mol Cell 2010;37:183-95. [Crossref] [PubMed]
- Roelands J, Hendrickx W, Zoppoli G, et al. Oncogenic states dictate the prognostic and predictive connotations of intratumoral immune response. J Immunother Cancer 2020;8:e000617. [Crossref] [PubMed]
- Katoh M, Katoh M. Transcriptional mechanisms of WNT5A based on NF-kappaB, Hedgehog, TGFbeta, and Notch signaling cascades. Int J Mol Med 2009;23:763-9. [Crossref] [PubMed]
- Douglass SM, Fane ME, Sanseviero E, et al. Myeloid-Derived Suppressor Cells Are a Major Source of Wnt5A in the Melanoma Microenvironment and Depend on Wnt5A for Full Suppressive Activity. Cancer Res 2021;81:658-70. [Crossref] [PubMed]
- Kim JE, Bang SH, Choi JH, et al. Interaction of Wnt5a with Notch1 is Critical for the Pathogenesis of Psoriasis. Ann Dermatol 2016;28:45-54. [Crossref] [PubMed]
- Deng Q, Li P, Che M, et al. Activation of hedgehog signaling in mesenchymal stem cells induces cartilage and bone tumor formation via Wnt/β-Catenin. Elife 2019;8:50208. [Crossref] [PubMed]
(English Language Editor: J. Jones)


