Analysis of influencing factors on the plasma concentration of first-line anti-tuberculosis drugs-a single-center retrospective cohort study
Introduction
According to a report from the World Health Organization (WHO), the annual incidence of tuberculosis (TB) is about 10 million worldwide and China accounts for 9% of all the cases globally. Although the total number of deaths from TB is decreasing globally, it remains one of the top 10 causes of death worldwide (1). Isoniazid (INH), rifampicin (RFP), ethambutol (EMB), and pyrazinamide (PZA) are the most commonly used first-line anti-TB drugs in clinical practice (2). The efficacy of anti-TB drugs is influenced by several factors, including the metabolic status of the tubercle bacilli in the lesion(3), drug resistance, comorbidities (4), drug dose, and serum drug concentration (5). Substandard plasma concentration can lead to bacterial drug resistance (6), disease relapse, or treatment failure (7). It has been reported that about 46% of TB patients failed to achieve sufficient serum concentration of at least one anti-TB drug, and the factors that contribute to substandard blood levels include drug dose, acetyl INH/INH ratio, and creatinine clearance (8). A small sample size (69 patients) (8), lack of multivariate analysis (only univariate analyses) (9,10) have been found in previous studies on the factors influencing the plasma concentration of anti-TB drugs. Factors affecting blood concentration of antituberculosis drugs have not been elaborated clearly.
The purpose of this study was to investigate the plasma concentration status of 4 first-line anti-TB drugs and to explore the determinants of anti-TB drug plasma concentration through multiple linear regression analysis, with an aim to guide rational clinical use, optimize individual patient doses, reduce the drug resistance of anti-TB drugs, and improve the cure rate of TB. We present the following article in accordance with the STROBE reporting checklist (11) (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1341/rc).
Methods
Study design
This single-center retrospective cohort study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) (12). Patient informed consent was waived due to the retrospective nature of the study, and the study was approved by the Medical Ethics Committee of the 309th Hospital of the Chinese People’s Liberation Army (PLA). (No. 201607110904). The Medical Support Center of the PLA General Hospital serves all medical centers affiliated, including 309 Hospital.
Study participants
Patients with pulmonary TB admitted to the 309th Hospital of the PLA from June 2014 to September 2018 were retrospectively studied. The inclusion criteria were as follows: (I) ≥16 years; (II) received 4 first-line anti-TB drugs (INH, RFP, EMB, PZA); and (III) taking drugs for at least 5 days before plasma concentration measurement. The exclusion criteria were as follows (met at least one): (I) human immunodeficiency virus (HIV) infection; (II) chronic gastrointestinal disease; (III) taken a compound preparation; (IV) frequent vomiting; or (V) missing important data.
Data collection
Patient demographic characteristics and clinical data were collected from the hospital electronic medical record system. The data collected included: patient demographic characteristics, admission diagnosis, comorbidities (diabetes, abnormal liver function, hyperuricemia, renal insufficiency), primary/recurrent treatment, nutritional status, major symptoms, drug dose, route of administration, plasma concentration at 2 hours after drug administration, biochemical parameters such as liver and kidney function before drug administration, and the use of drugs metabolized through the liver and renal.
The inclusion of the potential factors associated with anti-tuberculosis plasma concentration was based on clinical relevance and previous reports (8-10).
Study endpoints and definitions
The primary study endpoint was factors affecting the plasma concentration of anti-TB drugs. The secondary endpoint was the qualified rate of plasma concentration. All anti-TB drugs were administered on an empty stomach. Venous blood was drawn from patients 2 hours after dosing. Afterwards, the peak concentrations of the 4 drugs (INH, RMP, EMB, and PZA) were measured (13). Evidence from a previous study suggested that INH, RMP, EMB, and PZA reach peak serum concentrations approximately 2 hours after ingestion (13). According to the reference range recommended by the 2021 Expert Consensus on the Clinical Use of Drug Monitoring for Anti-TB Drug Therapy (14), low 2-hour blood levels are defined as follows: INH <3 µg/mL; RMP <8 µg/mL; PZA <20 µg/mL; and EMB <2 µg/mL.
Plasma concentration assay
The samples were analyzed by high performance liquid chromatography-mass spectrometry (15). The chromatographic conditions were as follows: an Agilent Poroshell 120SB C18 column (4.6 mm × 50 mm, 2.7 µm) was used as the analytical column, and a ZORBAX SB C18 column (2.1 mm × 12.5 mm, 5 µm) was used as the guard column with 0.2% methanol-0.2% acetic acid solution as the mobile phase, and gradient elution was conducted. The concentration of methanol was set at the following time settings: 0.01 min, 5%; 2.50 min, 17%; 5.00 min, 35%; 6.00 min, 70%; 6.01 min, 80%; 9.00 min, 90%; 9.01 min, 5%; 12.00 min, 5%. The flow rate was 0.5 mL·min-1 and the injected volume was 5 µL. The detection was performed by positive multiple reaction monitoring (MRM) with electrospray ionization (ESI). INH’s m/z 138.0→121.0, RFP’s m/z 823.2→791.2, EMB’s m/z 205.2→116.1, PZA’s m/z 124.1→79.0, and levofloxacin’s m/z 823.2→791.2. The mass spectrometry conditions were as follows: ion source: ion spray of ESI (ion spray); ion spray voltage (IS): 5,000 V; temperature of ion source (TEM): 350 ℃; in-source gas 1 (GS1, N2): 25 psi; in-source gas 2 (GS1, N2): 45 psi; curtain gas pressure (Cur Gas): 30 psi. Positive ion mode detection, MRM scanning mode, and other mass spectrometry parameters are shown in Table 1.
Table 1
| Name of drug | Retention time (tR/min) | Ion pair of MRM (m/z) | Optimized declustering potential in positive ion mode declustering potential (DP) | Focus potential entrance potential (EP) | Collision potential collision energy (CE) | Collision cell exit potential (CXP) |
|---|---|---|---|---|---|---|
| INH | 2.2 | 138.0→121.0 | 44.0 | 4 | 22 | 3 |
| REP | 8.4 | 823.2→791.2 | 62.0 | 7 | 29 | 7 |
| PZA | 4.0 | 124.1→79.0 | 37.0 | 4 | 23 | 3 |
| EMB | 1.2 | 205.2→116.1 | 38.5 | 4 | 21 | 3 |
TB, tuberculosis; MRM, multiple reaction monitoring; INH, isoniazid; RFP, rifampicin; PZA, pyrazinamide; EMB, ethambutol.
Statistical analysis
Continuous variables conforming to a normal distribution were expressed as mean ± standard deviation (mean ± SD), otherwise median (IQR) was used to express non-normally distributed variables, and comparisons between two groups were performed by the independent samples t-test and non-parametric Mann-Whitney U rank sum test, respectively. Categorical variables were expressed as percentages, and comparisons among groups were performed by the Chi-square test or Fisher’s exact probability test. The missing data were handled by mean imputation method. The comparisons were conducted between groups with different gender, whether or not initial therapy, and whether or not with comorbidities.
First, a univariate analysis was performed. Subsequently, multivariate analysis was conducted by using a multiple linear regression model (16). Multicollinearity test was performed before variables were included into the model (17), and variables with variance inflation factor (VIF) greater than 10 were excluded. Then, the variables were screened by stepwise selection, and the variables included in the final model were independent factors. A P value (two side) <0.05 was considered as statistically significant. All statistical analyses were performed by MedCalc Statistical Software version 19.0.4 (MedCalc Software bvba, Ostend, Belgium; https://www.medcalc.org; 2019).
Results
Baseline characteristics of the included patients
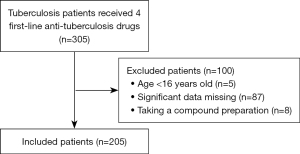
A total of 305 patients with pulmonary TB were treated with 4 first-line anti-TB drugs during the study period. Patients were screened in accordance with the inclusion and exclusion criteria, and 205 patients were finally enrolled (Figure 1). The median age of the included patients was 26 (IQR, 21–38.5) years, with 151 (73.7%) males and 31 patients (15.1%) on repeat treatment. The doses taken by the included patients are summarized in Table 1. The EMB median dose of 1 (IQR, 0.75–1) g did not reach the target dose range, while the median doses of the remaining 3 drugs were in the target range. The substandard rates of patients with 2-hour plasma concentrations for INH, rifampin, EMB, and PZA were 45.8%, 54.4%, 37.7%, and 52.9%, respectively. The baseline characteristics of the included patients are shown in Table 2.
Table 2
| Characteristics | Included patients (n=205) |
|---|---|
| Age (years) | 26 (21–38.5) |
| Male, n (%) | 151 (73.7) |
| Retreatment, n (%) | 31 (15.1) |
| With comorbidities, n (%) | 67 (32.7) |
| Drug dosage | |
| INH (g/d) | 0.4 (0.3–0.4) |
| RFP (g/d) | 0.6 (0.45–0.6) |
| EMB (g/d) | 1 (0.75–1) |
| PZA (g/d) | 1.5 (1.5–1.5) |
| Biochemical indicators before drug administration | |
| AST (U/L) | 16.8 (12.9–21.8) |
| ALT (U/L) | 15.3 (9.9–21.9) |
| ALB (g/L) | 39.2 (34.9–42.9) |
| Tb (μmol/L) | 10.8 (8.2–16.4) |
| Cr (μmol/L) | 66.3 (51–74.8) |
| BUN (mmol/L) | 4.1 (3.3–5.4) |
| UA (μmol/L) | 355 [274–444] |
| 2-hour plasma concentration | |
| INH (μg/mL) | 3.15 (2.03–4.29) |
| RFP (μg/mL) | 7.66±4.93 |
| EMB (μg/mL) | 2.21 (1.56–3.21) |
| PZA (μg/mL) | 19.64±9.14 |
Data are present as n (%)/median (IQR). INH, isoniazid; RFP, rifampicin; EMB, ethambutol; PZA, pyrazinamide; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALB, albumin; Tb, total bilirubin; Cr, creatinine; BUN, blood urea nitrogen; UA, uric acid.
Two-hour serum concentrations of anti-TB drugs and influencing factors
Isoniazid (INH)
The median plasma concentration of INH was 3.15 µg/mL (IQR, 2.03–4.29 µg/mL). The dose (P=0.002) and pre-dose blood uric acid level (P=0.007) were significantly associated with the 2-hour plasma concentrations of INH (Table 3) in the univariate analysis. Multivariate analysis demonstrated that intravenous administration (P<0.001) significantly increased plasma concentrations compared with oral administration. Plasma concentrations were negatively correlated with blood uric acid levels (P=0.0012), and the plasma concentrations of INH decreased by 0.002528 µg/mL for every 1 µmol/L increase in uric acid (Table 4).
Table 3
| Drug | Variable | Constant | Coefficient/Mann-Whitney U | F-ratio/test statistic Z | P value |
|---|---|---|---|---|---|
| INH | Male | 3,618 | 1.227 | 0.22 | |
| Age | 3.31 | 0.0439 | 0.238 | 0.62 | |
| Retreatment | 2,122 | 1.89 | 0.058 | ||
| Comorbidities | 5,071 | 0.426 | 0.66 | ||
| Malnutrition level | 3.41 | 0.046 | 0.045 | 0.83 | |
| Drug dosage | 1.79 | 4.35 | 9.642 | 0.002 | |
| Intravenous administration | 643.5 | 0.879 | 0.37 | ||
| ALT | 3.40 | 0.0011 | 0.03 | 0.86 | |
| AST | 3.42 | 0.0001567 | 0.001828 | 0.96 | |
| ALB | 3.86 | −0.01119 | 0.455 | 0.50 | |
| Cr | 3.45 | −0.0001978 | 0.1242 | 0.72 | |
| BUN | 3.51 | −0.01159 | 0.8325 | 0.36 | |
| UA | 4.27 | −0.002144 | 7.2002 | 0.007 | |
| Tb | 3.25 | 0.01264 | 0.7691 | 0.38 | |
| RFP | Male | 2,697.50 | 1.394 | 0.16 | |
| Age | 7.18 | 0.1987 | 0.4695 | 0.49 | |
| Retreatment | 1,337 | 0.577 | 0.56 | ||
| Comorbidities | 3,150 | 1.606 | 0.10 | ||
| Malnutrition level | 7.95 | −0.9621 | 2.523 | 0.11 | |
| Drug dosage | 5.35 | 4.3433 | 0.9586 | 0.32 | |
| Intravenous administration | 1,072 | 0.481 | 0.63 | ||
| ALT | 7.24 | 0.0239 | 0.5537 | 0.45 | |
| AST | 7.40 | 0.01412 | 0.1296 | 0.71 | |
| ALB | 3.92 | 0.09465 | 4.154 | 0.04 | |
| Cr | 7.96 | −0.003767 | 1.6558 | 0.19 | |
| BUN | 7.74 | −0.01512 | 0.07638 | 0.78 | |
| UA | 8.74 | −0.002887 | 1.4583 | 0.22 | |
| Tb | 8.23 | −0.04011 | 1.1820 | 0.27 | |
| EMB | Male | 5,079.00 | 0.929 | 0.35 | |
| Age | 2.00 | 0.2034 | 9.7893 | 0.002 | |
| Retreatment | 3,469.5 | 1.307 | 0.19 | ||
| Comorbidities | 6,292 | 1.018 | 0.3 | ||
| Malnutrition level | 2.53 | −0.03567 | 0.04921 | 0.82 | |
| Drug dosage | 1.30 | 1.3311 | 9.3394 | 0.002 | |
| ALT | 2.74 | −0.01218 | 3.1469 | 0.07 | |
| AST | 2.88 | −0.01888 | 4.3411 | 0.03 | |
| ALB | 3.36 | −0.02136 | 2.8786 | 0.09 | |
| Cr | 2.33 | 0.001587 | 14.2467 | <0.001 | |
| BUN | 2.36 | 0.02381 | 5.7296 | 0.01 | |
| UA | 2.39 | 0.0003420 | 0.3202 | 0.57 | |
| Tb | 2.4835 | 0.003170 | 0.09202 | 0.76 | |
| PZA | Male | 2,984 | 2.096 | 0.03 | |
| Age | 18.99 | 0.2633 | 0.3233 | 0.57 | |
| Retreatment | 2,105.50 | 1.771 | 0.07 | ||
| Comorbidities | 4,844.00 | 0.0974 | 0.92 | ||
| Malnutrition level | 20.01 | −1.2029 | 1.2855 | 0.25 | |
| Drug dosage | 1.84 | 12.4745 | 16.1062 | <0.001 | |
| ALT | 19.44 | 0.01134 | 0.05241 | 0.81 | |
| AST | 19.87 | −0.01325 | 0.04420 | 0.83 | |
| ALB | 18.89 | 0.01883 | 0.04160 | 0.83 | |
| Cr | 20.19 | −0.004356 | 2.7130 | 0.10 | |
| BUN | 20.81 | −0.1673 | 7.5994 | 0.006 | |
| UA | 22.15 | −0.006429 | 2.6766 | 0.10 | |
| Tb | 16.04 | 0.2712 | 12.6616 | <0.001 |
INH, isoniazid; RFP, rifampicin; EMB, ethambutol; PZA, pyrazinamide; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALB, albumin; Tb, total bilirubin; Cr, creatinine; BUN, blood urea nitrogen; UA, uric acid.
Table 4
| Drug | Independent variables | Coefficient | Std. error | t* | P value | rpartial | rsemipartial | VIF |
|---|---|---|---|---|---|---|---|---|
| INH | Constant | 4.3484 | ||||||
| Intravenous administration | 3.4495 | 0.7937 | 4.346 | <0.001 | 0.3007 | 0.2953 | 1.015 | |
| Uric acid | −0.002528 | 0.0007679 | −3.292 | 0.0012 | −0.2323 | 0.2237 | 1.015 | |
| RFP | Constant | 3.9292 | ||||||
| Albumin | 0.09465 | 0.04644 | 2.038 | 0.0430 | 0.1510 | 0.1510 | 1.000 | |
| EMB | Constant | 0.6107 | ||||||
| Age | 0.1538 | 0.06400 | 2.402 | 0.0170 | 0.1532 | 0.1453 | 1.053 | |
| Dose | 1.4680 | 0.4192 | 3.502 | <0.001 | 0.2205 | 0.2118 | 1.008 | |
| Creatinine | 0.001485 | 0.0004190 | 3.544 | <0.001 | 0.2230 | 0.2143 | 1.061 | |
| PZA | Constant | −0.7236 | ||||||
| Dose | 15.1382 | 2.9117 | 5.199 | <0.001 | 0.3451 | 0.3242 | 1.025 | |
| Retreatment | −3.4591 | 1.6620 | −2.081 | 0.0387 | −0.1456 | 0.1298 | 1.063 | |
| Gender | −3.6363 | 1.3734 | −2.648 | 0.0088 | −0.1840 | 0.1651 | 1.027 | |
| Urea nitrogen | −0.1818 | 0.05586 | −3.256 | 0.0013 | −0.2243 | 0.2030 | 1.029 | |
| Total bilirubin | 0.2535 | 0.07234 | 3.504 | <0.001 | 0.2405 | 0.2185 | 1.069 |
*, the t value is used to judge the significance of each independent variable. If significant, it indicates that the variable has a significant impact on the model. INH, isoniazid; RFP, rifampicin; EMB, ethambutol; PZA, pyrazinamide; VIF, variance inflation factor.
Rifampicin (RFP)
The mean plasma concentration of RFP was 7.66±4.93 µg/mL. Serum albumin levels (P=0.04) were significantly correlated with RFP plasma concentrations in the univariate analysis (Table 3). Multivariate analysis demonstrated that 2-hour plasma concentrations of RFP were positively correlated with serum albumin levels (P=0.043), for which plasma concentration of RFP increased 0.09465 µg/mL for every 1 g/L albumin increase (Table 4).
Ethambutol (EMB)
The median plasma concentration of EMB was 2.21 µg/mL (IQR, 1.56–3.21 µg/mL). Age (P=0.002), dose (P=0.002), aspartate transaminase (AST) (P=0.03), serum creatinine (P<0.001), and urea nitrogen (P=0.01) were significantly associated with the plasma concentrations of EMB in the univariate analysis (Table 3). The results of multivariate analysis suggested that the 2-hour plasma concentrations of EMB were positively correlated with age (P=0.017), dose (P<0.001), and serum creatinine level (P<0.001). The plasma concentration increased by 1.468 µg/mL for each 1 g increase in dose. Serum creatinine increased by 0.001485 µg/mL for each 1 µmol/L increase in plasma concentration (Table 4).
Pyrazinamide (PZA)
The mean plasma concentration of PZA was 19.64±9.14 µg/mL. Gender (P=0.03), dose (P<0.001), urea nitrogen (P=0.006), and total bilirubin (P<0.001) were significantly associated with plasma concentrations of PZA in the univariate analysis (Table 3). The results of the multivariate analysis demonstrated that plasma concentrations in retreated patients (P=0.0387) and male patients (P=0.0088) were significantly lower than in primary patients and female patients. PZA 2-hour plasma concentrations were positively correlated with dose (P<0.001) and total bilirubin levels (P<0.001). The plasma concentration increased by 15.1382 µg/mL for every 1 g dose increase, and the plasma concentration increased by 0.2535 µg/mL for every 1 µmol/L increase in total bilirubin level. The 2-hour plasma concentrations of PZA were negatively correlated with blood urea nitrogen levels (P=0.0013), for which the plasma concentration decreased by 0.1818 µg/mL for every 1 mmol/L increase in urea nitrogen level (Table 4).
Discussion
In this study, 205 patients with pulmonary TB treated with first-line anti-TB drugs were included, and the relationships between patient demographic characteristics and major biochemical indicators prior to drug administration and 2-hour plasma concentrations of anti-TB drugs were analyzed by a multiple linear regression model. The results showed that nearly half of the patients had a substandard plasma concentration of at least one drug. Univariate analysis revealed that age, gender, dose, and pre-dose levels of blood uric acid, serum albumin, AST, serum creatinine, urea nitrogen, and total bilirubin were significantly associated with anti-TB drug plasma concentrations. The multivariate analysis showed that age, gender, dose, intravenous administration, retreatment, blood uric acid level, serum albumin level, serum creatinine level, total bilirubin level, and blood urea nitrogen level were independent influencing factors for the plasma concentration of anti-TB drugs.
Relationship with previous studies
Rates of substandard blood concentration of anti-TB drugs
A 2016 meta-analysis (18) conducted a systematic review of 41 studies on 2-hour plasma concentrations of first-line anti-TB drugs, and the combined results showed that the substandard plasma concentration rates of INH, RFP, EMB, and PZA were 43% (95% CI: 32–55%), 67% (95% CI: 60–74%), 27% (95% CI: 17–38%), and 12% (95% CI: 7–19%), respectively. The results of our study are consistent with the above study. The above evidence suggests that substandard plasma concentration of first-line anti-TB drugs are currently not uncommon in clinical settings, which may lead to treatment failure, disease relapse, and drug resistance (13). Therefore, plasma concentration monitoring is essential to optimize the efficacy of anti-TB treatment(19).
Factors influencing the plasma concentration of anti-TB drugs
In a study conducted by Um et al. (8) in 2007, 69 patients with pulmonary TB were included and the factors influencing the 2-hour plasma concentration of anti-TB drugs were analyzed by multiple linear regression models. The results showed that drug dose was positively correlated with the plasma concentration, which is consistent with the results of the present study, indicating that the insufficient dose is an important factor that leads to substandard blood drug concentration. INH is generally administered orally. However, it can be administered intramuscularly or intravenously in critically ill patients (20). Our study found that intravenous administration significantly increased plasma concentrations compared with oral administration, which may be attributed to the effect of food and the first-pass effect of the liver (21). A large number of previous studies have shown that anti-TB drugs can affect uric acid metabolism in humans, which could result in elevated blood uric acid (22-24). The mechanisms of the interaction between blood uric acid and anti-TB drug metabolism need to be further investigated.
The study showed a positive correlation between RFP plasma concentrations and serum albumin levels, which may be attributed to the albumin binding rate of the drug. A previous study had reported an albumin binding rate of 70–80% for RFP (25), which was higher than other first-line anti-TB drugs. These results suggest that serum albumin is an important factor affecting the serum concentration of RFP, most likely because hypoalbuminemia leads to more clearance of unbound RFP by the liver (26). The relationship between albumin and RFP plasma concentrations in this study is consistent with a previous study (27), and is also in line with the results of a pharmacokinetic study for RFP (28). Future studies are warranted to focus on the relationship between serum total drug concentration and free drug concentration of RFP.
In a 2007 study by Um et al. (8), the 2-hour plasma concentration of EMB was significantly correlated with age, dose, and creatinine clearance. In 2019, the results of a study from China confirmed that the plasma concentration of EMB was significantly correlated with dose and creatinine clearance in the univariate and multivariate analyses(27). These results are consistent with our findings in the present study. EMB is a drug excreted through the kidneys, and its excretion decreases concurrently when creatinine clearance decreases, which may explain the negative correlation between its plasma concentration and creatinine clearance. A previous study had shown a positive correlation between the plasma concentration of EMB and age, which is consistent with the present study. This may be caused by metabolic and excretory capacity decreases with age, which can result in a slower decrease in plasma concentration (29). Therefore, the dose of EMB can be appropriately reduced in clinical practice to maintain plasma concentrations in the target range in patients with decreased creatinine clearance or in the elderly.
Two previous studies indicated that the 2-hour plasma concentrations of PZA were significantly higher in female patients than in male patients (8,27), which is consistent with the results of the present study. The mechanism of the discrepancy in plasma concentration of anti-tuberculosis drugs between genders needs further studies. The univariate and multivariate analyses in this study showed that plasma concentrations of PZA were positively correlated with dose and total serum bilirubin levels, which was consistent with previous report (29). When total bilirubin levels are elevated, it indicates that the liver is less able to clear both bilirubin and anti-TB drugs, so blood drug concentration levels may be increased concurrently. When liver function was impaired, PZA doses could be reduced appropriately. Blood urea nitrogen was elevated in response to impaired renal function, which was similar to serum creatinine, while 4–14% of PZA is excreted in urine in prototype form after administration (30), and this may account for the negative correlation between plasma concentrations of PZA and blood urea nitrogen.
Limitations of the study
First, this was a retrospective study. Therefore, there was an insufficient causal relationship between the included variables and drug concentrations, which should be validated in a prospective randomized controlled study. Second, the study excluded a large number of patients with missing data and only analyzed patients with complete data, which may lead to bias. Third, this study did not analyze the relationship between drug concentration, efficacy and patients prognosis, so future studies in this field should expand the endpoints. Fourth, a single time point of 2 hours was used for the determination of plasma concentrations in the study, which may miss the actual peak concentration. Lastly, this single-center study did not have sufficient generalizability of the findings.
Conclusions
In clinical practice, there is a high rate of substandard blood concentration of first-line anti-TB drugs at 2 h post-administration. The multivariate analysis showed that age, gender, dose, intravenous administration, retreatment, blood uric acid level, serum albumin level, serum creatinine level, total bilirubin level, and blood urea nitrogen level were independent influencing factors for anti-TB drug plasma concentration. During anti-TB treatment, plasma concentration monitoring is essential and helpful to optimize the drug dose and carry out individualized treatment regimen, so as to effectively improve the therapeutic effect and reduce side effects.
Acknowledgments
Funding: This work was supported by the Capital Clinical Characteristic Application Research of China (No. Z141107002514023).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1341/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1341/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1341/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Medical Ethics Committee of the 309th Hospital of the Chinese People’s Liberation Army (PLA) (No. 201607110904) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Floyd K, Glaziou P, Zumla A, et al. The global tuberculosis epidemic and progress in care, prevention, and research: an overview in year 3 of the End TB era. Lancet Respir Med 2018;6:299-314. [Crossref] [PubMed]
- Furin J, Cox H, Pai M. Tuberculosis. Lancet 2019;393:1642-56. [Crossref] [PubMed]
- Evangelopoulos D, da Fonseca JD, Waddell SJ. Understanding anti-tuberculosis drug efficacy: rethinking bacterial populations and how we model them. Int J Infect Dis 2015;32:76-80. [Crossref] [PubMed]
- Saito N, Yoshii Y, Kaneko Y, et al. Impact of renal function-based anti-tuberculosis drug dosage adjustment on efficacy and safety outcomes in pulmonary tuberculosis complicated with chronic kidney disease. BMC Infect Dis 2019;19:374. [Crossref] [PubMed]
- Rangaka MX, Cavalcante SC, Marais BJ, et al. Controlling the seedbeds of tuberculosis: diagnosis and treatment of tuberculosis infection. Lancet 2015;386:2344-53. [Crossref] [PubMed]
- Dheda K, Lenders L, Magombedze G, et al. Drug-Penetration Gradients Associated with Acquired Drug Resistance in Patients with Tuberculosis. Am J Respir Crit Care Med 2018;198:1208-19. [Crossref] [PubMed]
- Weiner M, Burman W, Vernon A, et al. Low isoniazid concentrations and outcome of tuberculosis treatment with once-weekly isoniazid and rifapentine. Am J Respir Crit Care Med 2003;167:1341-7. [Crossref] [PubMed]
- Um SW, Lee SW, Kwon SY, et al. Low serum concentrations of anti-tuberculosis drugs and determinants of their serum levels. Int J Tuberc Lung Dis 2007;11:972-8. [PubMed]
- Sharma PK, Bansal R, Bhardwaj AK, et al. Plasma levels of Rifampicin and Pyrazinamide with pre and post meal administration in tuberculosis patients. Indian J Tuberc 2018;65:35-40. [Crossref] [PubMed]
- Tersigni C, Boiardi G, Tofani L, et al. Real-life isoniazid and rifampicin plasma concentrations in children: a tool for therapeutic drug monitoring of tuberculosis. BMC Infect Dis 2021;21:1087. [Crossref] [PubMed]
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg 2014;12:1495-9. [Crossref] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191-4. [Crossref] [PubMed]
- Alsultan A, Peloquin CA. Therapeutic drug monitoring in the treatment of tuberculosis: an update. Drugs 2014;74:839-54. [Crossref] [PubMed]
- Lu Y, Zhu H. Expert consensus on the therapeutic drug monitoring of anti-tuberculosis drugs. Chinese Journal of Antituberculosis 2021;43:867-73.
- Song SH, Jun SH, Park KU, et al. Simultaneous determination of first-line anti-tuberculosis drugs and their major metabolic ratios by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 2007;21:1331-8. [Crossref] [PubMed]
- Hu YH, Yu SC, Qi X, et al. An overview of multiple linear regression model and its application. Zhonghua Yu Fang Yi Xue Za Zhi 2019;53:653-6. [PubMed]
- Llorca J. Omission of variables in regression models with high multi-colinearity. Gac Sanit 1999;13:243-4. [Crossref] [PubMed]
- Mota L, Al-Efraij K, Campbell JR, et al. Therapeutic drug monitoring in anti-tuberculosis treatment: a systematic review and meta-analysis. Int J Tuberc Lung Dis 2016;20:819-26. [Crossref] [PubMed]
- Verbeeck RK, Günther G, Kibuule D, et al. Optimizing treatment outcome of first-line anti-tuberculosis drugs: the role of therapeutic drug monitoring. Eur J Clin Pharmacol 2016;72:905-16. [Crossref] [PubMed]
- WHO Guidelines Approved by the Guidelines Review Committee. Treatment of Tuberculosis: Guidelines. Geneva: World Health Organization. Copyright © 2010, World Health Organization, 2010.
- Erwin ER, Addison AP, John SF, et al. Pharmacokinetics of isoniazid: The good, the bad, and the alternatives. Tuberculosis (Edinb) 2019;116S:S66-70. [Crossref] [PubMed]
- Ha YJ, Chung SW, Lee JH, et al. Clinical features and risk factors for gout attacks during anti-tuberculosis treatment: A case-control study in South Korea. Int J Rheum Dis 2019;22:1905-11. [Crossref] [PubMed]
- Louthrenoo W, Hongsongkiat S, Kasitanon N, et al. Effect of Antituberculous Drugs on Serum Uric Acid and Urine Uric Acid Excretion. J Clin Rheumatol 2015;21:346-8. [Crossref] [PubMed]
- Thumamo Pokam BD, Enoh JE, Eyo AO, et al. Uric acid levels in patients on antituberculosis drugs in the southwest Region of Cameroon. Int J Mycobacteriol 2018;7:89-91. [Crossref] [PubMed]
- Woo J, Cheung W, Chan R, et al. In vitro protein binding characteristics of isoniazid, rifampicin, and pyrazinamide to whole plasma, albumin, and alpha-1-acid glycoprotein. Clin Biochem 1996;29:175-7. [Crossref] [PubMed]
- Tappero JW, Bradford WZ, Agerton TB, et al. Serum concentrations of antimycobacterial drugs in patients with pulmonary tuberculosis in Botswana. Clin Infect Dis 2005;41:461-9. [Crossref] [PubMed]
- Lei Q, Wang H, Zhao Y, et al. Determinants of serum concentration of first-line anti-tuberculosis drugs from China. Medicine (Baltimore) 2019;98:e17523. [Crossref] [PubMed]
- Abulfathi AA, Decloedt EH, Svensson EM, et al. Clinical Pharmacokinetics and Pharmacodynamics of Rifampicin in Human Tuberculosis. Clin Pharmacokinet 2019;58:1103-29. [Crossref] [PubMed]
- McIlleron H, Wash P, Burger A, et al. Determinants of rifampin, isoniazid, pyrazinamide, and ethambutol pharmacokinetics in a cohort of tuberculosis patients. Antimicrob Agents Chemother 2006;50:1170-7. [Crossref] [PubMed]
- Pyrazinamide. Tuberculosis (Edinb) 2008;88:141-4. [Crossref] [PubMed]
(English Language Editor: C. Betlazar-Maseh)