
Cost-effectiveness analysis of vonoprazan versus proton pump inhibitors in the treatment of reflux esophagitis in China
Introduction
Reflux esophagitis (RE) is an esophageal mucosal injury that occurs secondary to retrograde flux of gastric contents into the esophagus and referred as one of phenotypes of gastroesophageal reflux disease (GERD). It is considered a common disease worldwide with increasing prevalence. The estimated prevalence of RE in China was 6.4% based on a population-based study, of whom 3% had sever diseases categorized as Los Angeles (LA) grades C/D (1). The typical symptoms of RE include heartburn and/or regurgitation. Patients may also have other symptoms such as epigastric pain or sleep disturbance, which subsequently affect their quality of life (QOL) (2). Anxiety and depression levels were also significantly higher in people with reflux symptoms, which could result in reduced work productivity and poses a great burden on the society (3).
The main treatment goals of RE are to heal the breaking mucosal and relieve symptoms, as well as to prevent complications and improve QOL (4). Current guidelines recommended proton pump inhibitors (PPIs) for 8 weeks as an initial treatment for RE patients. Once healing of mucosal erosions and symptom relief have been achieved by initial therapy, long-term maintenance treatment with the lowest effective dose of PPIs is also recommended (4-6). In China, approved PPI treatment for RE includes omeprazole (OME), esomeprazole (ESO), rabeprazole (RAB), pantoprazole (PAN), lansoprazole (LAN) and ilaprazole (ILAP). However, PPIs have notable limitations: they do not provide complete acid control and exhibit nocturnal acid breakthrough. A nationwide survey demonstrated that over 80% of adults taking PPIs for reflux diseases reported nocturnal symptoms, which affected their QOL (7). The healing rate with PPIs is also low. A previous study has reported that approximately 4–15% of RE patients fail to achieve complete healing esophageal inflammation after the 8-week standard-dose PPI treatment (8). Also, available evidence shows that a considerable number of patients relapse during maintenance PPI treatment (9).
Vonoprazan (VPZ) is a potassium-competitive acid blocker (P-CAB) for the treatment of gastric acid-related diseases. It has been approved in China for RE treatment since 2019. VPZ exerts faster, more potent and more sustainable acid inhibitory effects than PPIs due to its excellent pharmacological characteristics (10,11). A 4-week treatment with VPZ and 8-week treatment with a PPI has been recommended as initial therapies for RE patients (12). A phase III study found that the mucosal healing rate of the VPZ 20 mg group at week 4 was higher than that in the LAN 30 mg group at week 8 (at 4 weeks 96.1% vs. 90.9%, P<0.05; at 8 weeks 98.9% vs. 94.5%, P<0.03) (13). Another phase III study based on an Asian population in which Chinese patients comprised >50% of the total study population, also reported non-inferiority between of VPZ 20 mg and LAN 30 mg based on the endoscopic erosion healing rate at week 8, and the incidence of treatment-emerging adverse events was similar between the VPZ 20 mg group and LAN 30 mg group (38.1% vs. 36.6%, respectively) (14).
As of now, only three cost-effectiveness of VPZ in the treatment of RE have been published (15-17). However, all these studies evaluated the cost-effectiveness of VPZ from Japanese healthcare payer’s perspective and found that VPZ is a cost-effective treatment compared with target PPI. Due to the healthcare system is significantly different between Japan and China, and the commonly used PPI is also different between these two countries, these make them of little use in terms of obtaining plausible conclusions for patients in China. Meanwhile, VPZ along with 70 other innovative drugs were successfully incorporated into the China National Reimbursement Drug List (NRDL) in December 2020. The daily costs for PPIs and VPZ were close. Thus, health economic evidence balancing efficacy and cost will play an important role to inform clinical medication choice and hospital listing. Therefore, this study aimed to estimate the cost-effectiveness of VPZ compared with all available PPIs for the treatment of RE patients in China from the healthcare system perspective. We present the following article in accordance with the CHEERS reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1722/rc).
Methods
Model development
A Markov model was developed in Microsoft Excel to predict the effectiveness and costs of VPZ versus other PPIs for RE treatment in China (18). Markov models are well studied for modeling the progression of chronic diseases and have been widely used in economic evaluations of GERD treatment (15,19-21). The model incorporated two treatment strategies: VPZ and a group of PPIs including OME, PAN, ESO, RAB, LAN and ILAP. Based on the results of published pairwise meta-analyses, there is no significant difference in the efficacy among different PPIs (22). Therefore, we chose a PPI group, rather than any single PPI, as the comparator.
We defined four disease states to represent possible consequences of RE treatment: mucosa healed, mucosa unhealed, relapse and death. A numeric QOL value and direct medical costs were assigned for each health states. QOL was calculated by quality-adjusted life years (QALYs), which is widely acknowledged as a measure of health outcome for economic assessment. A mean age of 40 years was used as the starting age of patients entering the model (1,23). The time horizon was 5 years and we used a 4-week cycle length (17) with half-cycle correction to accommodate the gradual transition of the population between health states. A discount rate of 5% (24) was applied for QALY and costs.
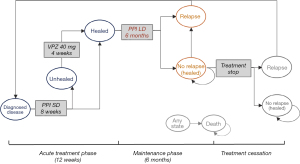
The model included an acute treatment phase and a maintenance phase, which reflects the general treatment pattern of all RE patients, as well as RE patients with moderate-to-severe disease. The flows of treatments, doses, and length of treatment for different strategies were based on the Chinese clinical practice guideline for GERD (5) and verified with clinical experts’ opinions.
Acute treatment and maintenance phase
The simulated cohort in the PPI strategy started receiving a standard-dose PPI for 8 weeks. If patients were not healed after initial treatment, continuous therapy on VPZ 40 mg once daily for an additional 4 weeks was added. After that, all patients were assumed to achieve endoscopically confirmed mucosal healing (25). The acute treatment phase lasted a maximum of 12 weeks. For patients healed at week 8 or 12, 6-month maintenance treatment with low-dose PPI was instituted.
The model structure of the VPZ strategy was slightly different. The simulated cohort of patients was initially treated with VPZ 20 mg once daily for 4 weeks. Patients who were not healed at week 4 continued on VPZ 20 mg once daily for an additional 4 weeks. For unhealed patients at week 8, VPZ 40 mg/day was given for 4 weeks to achieve mucosal healing. Patients who were healed at week 4, 8 and week 12 would progress to 6-month maintenance treatment with VPZ 10 mg.
Treatment cessation and relapse
Patients could stop treatment and remained in the “mucosa healed” state if they completed maintenance therapy without relapse. However, after initial healing of esophageal inflammation, 50–80% of RE patients experience relapse within 6 to 12 months after treatment cessation (26). Patients may experience multiple relapses. In all evaluated strategies, it was assumed that relapsed patients were reintroduced to acute treatment and remained in the same state. Although RE is associated with a low mortality rate (27), we still modeled death as a terminal state in the patient journey. The model structures of the PPI and VPZ strategies are shown in Figures 1,2 respectively.
Clinical inputs and transition probabilities
Clinical inputs of this model included (I) healing rates measured by the proportion of patients with endoscopically confirmed healing at weeks 4 and 8 during the acute treatment phase, and (II) relapse rates measured by the proportion of patients with endoscopically confirmed relapse during the 6-month maintenance phase. To measure the treatment efficacy of the PPI and VPZ groups, we conducted a systematic review with a series of meta-analyses.
Systematic review
Search Strategy and Eligible Criteria. A structured search was conducted up to March 2019 in PubMed, Cochrane Library, China National Knowledge Infrastructure and WanFang to identify randomized controlled trials of PPIs and VPZ for RE treatment. The population of interest was adult patients with diagnosed RE. The healing and relapse rates had to be based endoscopically confirmed results. Full search strategies and the population, intervention, comparators, outcomes and study design criteria for inclusion in this systematic review are presented in the supplementary files (Tables S1,S2).
Data Extraction and Quality Assessment. Two investigators (SQ and RS) independently extracted all data, which were subsequently validated by a third independent reviewer (YS). All the included studies were critically appraised using a comprehensive assessment criterion based on the recommendations in the Cochrane Handbook (28).
Meta-analyses
For studies meeting the inclusion criteria, the number of patients healed or relapsed at each time interval and the number of patients initially at risk (i.e., intent-to-treat principle) were extracted.
We performed a single-arm meta-analysis for rates of healing and relapse by different treatment strategies, using the metaprop package in R software version 3.4 to obtain the pooled estimate of treatment effects of the PPI and VPZ groups. Logit transformation was implemented to normalize the distribution of a single rate calculated based on raw data before calculating the overall rate (29). Heterogeneity among the studies was assessed using the Chi-square test and measured by the I2 statistic. If the test for heterogeneity was not statistically significant (i.e., P<0.05, I2<25%), a fixed-effects model was assumed for estimating the pooled rates of healing or relapse and the 95% confidence intervals (CI) for different treatment strategies. Otherwise, a random-effects model was used.
Transition probability
The meta-analysis measured the pooled healing rate at weeks 4 and 8, and the relapse rate in the 6-month maintenance phase, which was used to calculate the transition probability corresponding to a Markov cycle length of 4 weeks as follows: P= 1 – exp(–rt), where e = event rates and t = time (30). Background mortality was considered in this model. The age- and sex-adjusted all-cause death rates of the population aged over 40 years were extracted from the China life table as inputs (31).
Cost and utility
The analysis was conducted from a healthcare system perspective, thus only direct medical costs of patients were considered, including drug costs, outpatient treatment costs, and laboratory test costs. The PPI group’s drug cost was calculated based on average tender prices of each PPI available in China weighted by corresponding market shares. We only considered the prices of branded PPIs. The average tender prices of each PPI were extracted from the YAOZHI® database. Market shares were collected from a panel of nationwide hospital surveys (n=12). Other medical expenses were estimated via local clinical expert interviews (n=10). Detailed calculation methods and interview results are presented in the supplementary material (Tables S3,S4). All costs are expressed in US dollars (USD) using an exchange rate of 1 CNY =0.145 USD, which was the average in 2020.
Utility was derived from a cross-sectional survey, using the five-level EuroQol five-dimensional questionnaire (EQ-5D-5L) to elicit preferences for RE patients in China (32). Utility for unhealed RE patients was 0.86. For severe RE patients, the utility was 0.69.
Sensitivity analysis
One-way sensitivity analysis (OWSA) was performed to test the robustness of the study results. We varied the healing/relapse rates, drug costs, utilities, and the discount rate according to the 95% CI for each value or by ±20% if the 95% CI was not available/estimable for the OWSA.
Probabilistic sensitivity analysis (PSA) with 1,000-time Monte-Carlo simulations was also conducted, with a gamma distribution being assigned for cost parameters and a beta distribution being assigned for utilities and transition probabilities, PSA allows all model variables to be varied simultaneously within a plausible range to estimate the probability that the intervention in question is cost-effective at different willingness to pay (WTP) thresholds.
A scenario analysis was undertaken to investigate the cost-effectiveness of VPZ and the PPI group in treating severe RE patients with Los Angeles grade C/D (LA C/D).
Results
Clinical inputs and transition probabilities
Systematic review and meta-analysis
We screened the titles of 911 potentially eligible studies. After de-duplication, abstract and full-text screening, we included 56 studies in the systematic review (Figure S1). The data extraction and risk of bias assessment for the included studies is presented in the supplementary files (Tables S5-S7, Figures S2,S3).
There were 38 studies (65 arms) with 24,020 RE patients with reported endoscopic healing rates for PPI treatment or VPZ treatment. Meta-analysis showed that rates of healing at weeks 4 and 8 tended to be higher in those who receiving VPZ 20 mg compared with those receiving standard-dose PPIs. At week 4, the healing rate with VPZ treatment and PPI treatment was 90% (95% CI: 82–97%) and 74% (95% CI: 71–76%), respectively. After 8-week treatment of RE, the healing rate was 94% (95% CI: 88–99%) for VPZ and 87% (95% CI: 85–88%) for the PPI group.
VPZ was also found to be more effective in patients with moderate-to-severe RE. We included 17 studies that reported endoscopic healing rates of 3,398 patients with moderate-to-severe RE categorized as LA C/D. Results showed that at week 4, the healing rate of patients with LA C/D was 90% (95% CI: 81–100%) for VPZ and 61% (95% CI: 55–67%) for the PPI group. At week 8, the healing rate of patients with LA C/D was 96% (95% CI: 90–100%) for VPZ and 79% (95% CI: 75–83%) for the PPI group.
There were 17 studies that evaluated the efficacy of low-dose PPIs as maintenance therapy for healed RE. Results showed that during 6-month maintenance therapy, 82% (95% CI: 80–85%) of all patients and 71% (95% CI: 65–77%) of LA C/D patients remained healed when treated with low-dose PPIs. Therefore, the relapse rate of all patients and those with LA C/D treated with PPIs was 18% and 29%, respectively. There was only one study that reported rates of RE recurrence following treatment with VPZ 10 mg: 5.1% for all patients and 13.2% for patients with LA C/D during 6-month maintenance therapy (33). Detailed meta-analysis results are summarized in the supplementary files (Figures S4-S13).
Transition probability
We converted the event rates (r) over a time period (t) to transition probabilities (p) using the formula p = 1 – exp(–rt). The estimated healing probabilities and relapse probabilities at different time points are presented in Table 1.
Table 1
| Phase and population | Parameters | VPZ 20 mg QD | PPI standard dose QD | VPZ 10 mg QD | PPI low dose QD | Treatment cessation |
|---|---|---|---|---|---|---|
| Healing rate during healing therapy | ||||||
| All RE patients | Healing rate at week 4 | 90% | 74% | |||
| Healing rate at week 8 | 94% | 87% | ||||
| Healing rate at weeks 4–8* | 40% | 50% | ||||
| LA C/D RE patients | Healing rate at week 4 | 90% | 61% | |||
| Healing rate at week 8 | 96% | 79% | ||||
| Healing rate at weeks 4–8* | 60% | 46% | ||||
| Relapse rate during maintenance therapy | ||||||
| All RE patients | Relapse rate in 6 months | 5% | 18% | |||
| Relapse rate in 4 weeks | 0.87% | 3.25% | ||||
| LA C/D RE patients | Relapse rate in 6 months | 13% | 29% | |||
| Relapse rate in 4 weeks | 2.33% | 5.55% | ||||
| Relapse rate after treatment cessation | ||||||
| All RE patients and LA C/D RE patients | Relapse rate in 6 months | 80% [9] | ||||
| Relapse rate in 4 weeks | 23.5% | |||||
*, healing rate at weeks 4–8 calculated by (healing rate at week 8 – healing rate at week 4)/(1 – healing rate at week 4). LA C/D, as Los Angeles grade C/D; PPI, proton pump inhibitors; QD, once daily; RE, reflux esophagitis; VPZ, vonoprazan.
Cost and health resource utilization
Drug costs and other medical expenses associated with RE treatment are listed in Table 2. The drug cost for VPZ used in the model was the price after NRDL negotiation updated in December 2020. The initiation of acute treatment included one outpatient visit and several follow-up visits every 2 weeks for drug prescription. During 6-month maintenance treatment, the outpatient visit was made every 4 weeks. Patients were required to undergo endoscopy at the first outpatient visit for diagnosis. For patients who were unhealed after the 8-week treatment, esophageal manometry and 24-hour pH monitoring were initiated.
Table 2
| Medical expense | Items | Cost (USD) | Notes |
|---|---|---|---|
| Drug cost (cost per cycle) | PPI group (standard dose, QD) | 65 | Unit cost ×28 days* |
| VPZ 20 mg QD | 40 | ||
| PPI group (low dose, QD) | 34 | ||
| VPZ 10 mg QD | 20 | ||
| Outpatient visit | Visit during healing phase | 15 | Twice a month |
| Visit during maintenance phase | 7 | Once a month | |
| Endoscopy | For diagnosis | 84 | Once |
| 24-hour pH monitoring | For patients who were unhealed after 8-week treatment | 116 | Once |
*, cycle costs for PPI group and VPZ can be calculated by multiplying the unit cost by 28 days. PPI, proton pump inhibitors; QD, once daily; VPZ, vonoprazan.
Base-case analysis
Over the 5-year time horizon, a 40-year-old RE patient treated with VPZ was associated with 0.02 QALYs gained and a cost saving of USD943 compared with the PPI group. Therefore, VPZ appears to be a dominant strategy compared with PPIs (more QALYs gained and less cost incurred) (Table 3).
Table 3
| Scenario | QALYs | ΔQALYs | Cost (USD) | ΔCost(USD) | ICER(USD) |
|---|---|---|---|---|---|
| Base case | |||||
| PPI group | 4.33 | 2,297 | |||
| VPZ | 4.35 | 0.02 | 1,354 | –943 | Dominant |
| Scenario analysis–LA C/D patients | |||||
| PPI group | 4.19 | 2,288 | |||
| VPZ | 4.27 | 0.08 | 1,352 | –936 | Dominant |
Δ, represents the difference between the two groups. ICER, incremental cost-effectiveness ratios; LA C/D, Los Angeles (LA) grade C/D; PPI, proton pump inhibitor; QALY, quality-adjusted life year; VPZ, vonoprazan.
Scenario analysis
The scenario analysis showed VPZ was also a cost-saving option compared with the PPI group for LA C/D patients (Table 3).
Sensitivity analysis
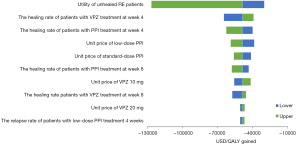
Results of OWSA are presented as a tornado diagram (Figure 3). VPZ remained cost-saving under each scenario investigated. Utility of unhealed RE patients, and the healing rate at week 4 for the VPZ and PPI groups had the greatest effects on the result.
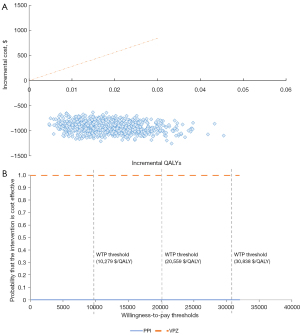
Results of PSA are summarized as a scatter plot in Figure 4A. The acceptability curve in Figure 4B shows that the likelihood of VPZ being considered cost-saving at a WTP threshold of USD30,838 per QALY gained was 100% compared with the PPI group.
Discussion
Several clinical trials have demonstrated that compared with other PPIs that available for RE patients, VPZ, a novel P-CAB, can provide rapid and sustained acid inhibition with good safety profile (13,14,33). However, no systematic comparison of the clinical effects and cost-effectiveness of VPZ and PPIs has been reported to date. This study, to the best of our knowledge, is the first to assess the treatment of RE patients in China.
The model we used incorporated both acute treatment and maintenance phases, which reflects current guidelines and treatment patterns in China. Our results demonstrated that treating RE with VPZ is an efficacious and cost-saving option compared with conventional PPIs. Subgroup analysis results further demonstrated that treatment with VPZ is cost-saving for patients with severe esophagitis. These results are in line with previous studies. A study in Japan evaluated the cost-effectiveness of VPZ versus LAN for the acute treatment of RE (15). It demonstrated that VPZ was consistently superior to LAN in terms of cost-effectiveness and medication duration. Another Japanese study evaluated the long-term cost and effectiveness of a VPZ-first strategy compared with the ESO-first and RAB-first strategies. Results showed that the VPZ-first strategy increased QALYs and appeared to be cost-effective for GERD patients compared with the ESO- or RAB-first strategy (17). Our findings now supersede these and indicated the superiority of VPZ over the PPI group in treating and maintaining RE.
Some limitations of our analysis should be noted. First, there is no head-to-head trial that has compared VPZ with most PPIs except LAN, and there is not a single trial comparing all available PPIs. Therefore, we used meta-analysis with further assumptions to combine data. However, most studies included in meta-analyses do not have a Chinese cohort. To make sure the pooled estimates can reflect treatment efficacy of Chinese patients all data inputs were validated by local clinical experts. Furthermore, sensitivity analyses were performed to access uncertainties. Second, we did not include the costs of adverse events in the analysis due to lack of data. Nonetheless, the costs associated with adverse events would not be an influential or differentiating feature of this study. Third, although generic PPIs have been used in RE treatment in clinical practice in China, we only considered original PPIs. Whether generic drugs have the same quality, therapeutic effect, and safety profile as the original drugs is a matter of concern.
Conclusions
In the current setting of the Chinese healthcare system, our analysis suggested that VPZ could be a cost-saving strategy in the treatment of RE patients in China. The findings of this study, which were based on local data, can inform treatment decision makers at both the level of the individual patient and the policy level.
Acknowledgments
Funding: This study was funded by Takeda (China) International Trading Company.
Footnote
Reporting Checklist: The authors have completed the CHEERS reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1722/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1722/coif). The authors report that this study was funded by Takeda (China) International Trading Company. RS and SQ are employees of IQVIA (China). YS and LD are employees of Takeda (China) International Trading Co. Ltd. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zou D, He J, Ma X, et al. Epidemiology of symptom-defined gastroesophageal reflux disease and reflux esophagitis: the systematic investigation of gastrointestinal diseases in China (SILC). Scand J Gastroenterol 2011;46:133-41. [Crossref] [PubMed]
- Vakil N, van Zanten SV, Kahrilas P, et al. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006;101:1900-20; quiz 1943. [Crossref] [PubMed]
- Choi JM, Yang JI, Kang SJ, et al. Association Between Anxiety and Depression and Gastroesophageal Reflux Disease: Results From a Large Cross-sectional Study. J Neurogastroenterol Motil 2018;24:593-602. [Crossref] [PubMed]
- Iwakiri K, Fujiwara Y, Manabe N, et al. Evidence-based clinical practice guidelines for gastroesophageal reflux disease 2021. J Gastroenterol 2022;57:267-85. [Crossref] [PubMed]
- Xiao YL, Zhou LY, Hou XH, et al. Chinese expert consensus on gastroesophageal reflux disease in 2020. J Dig Dis 2021;22:376-89. [Crossref] [PubMed]
- Katz PO, Dunbar KB, Schnoll-Sussman FH, et al. ACG Clinical Guideline for the Diagnosis and Management of Gastroesophageal Reflux Disease. Am J Gastroenterol 2022;117:27-56. [Crossref] [PubMed]
- Chey WD, Mody R, Chen L, et al. M1033 Nighttime Symptoms and Sleep Impairment Among Patients with Gastro-Esophageal Reflux Disease (GERD) Receiving Prescription (Rx) Proton Pump Inhibitors (PPIs). Gastroenterol 2008;134.
- Fass R, Sifrim D. Management of heartburn not responding to proton pump inhibitors. Gut 2009;58:295-309. [Crossref] [PubMed]
- Dickman R, Maradey-Romero C, Gingold-Belfer R, et al. Unmet Needs in the Treatment of Gastroesophageal Reflux Disease. J Neurogastroenterol Motil 2015;21:309-19. [Crossref] [PubMed]
- Matsukawa J, Inatomi N, Nishida H, et al. Pharmacological characteristics and clinical efficacies of a novel potassium-competitive acid blocker, vonoprazan fumarate. Nihon Yakurigaku Zasshi 2018;152:104-10. [Crossref] [PubMed]
- Oshima T, Miwa H. Potent Potassium-competitive Acid Blockers: A New Era for the Treatment of Acid-related Diseases. J Neurogastroenterol Motil 2018;24:334-44. [Crossref] [PubMed]
- Mori H, Suzuki H. Role of Acid Suppression in Acid-related Diseases: Proton Pump Inhibitor and Potassium-competitive Acid Blocker. J Neurogastroenterol Motil 2019;25:6-14. [Crossref] [PubMed]
- Ashida K, Sakurai Y, Hori T, et al. Randomised clinical trial: vonoprazan, a novel potassium-competitive acid blocker, vs. lansoprazole for the healing of erosive oesophagitis. Aliment Pharmacol Ther 2016;43:240-51. [Crossref] [PubMed]
- Xiao Y, Zhang S, Dai N, et al. Phase III, randomised, double-blind, multicentre study to evaluate the efficacy and safety of vonoprazan compared with lansoprazole in Asian patients with erosive oesophagitis. Gut 2020;69:224-30. [Crossref] [PubMed]
- Habu Y. Vonoprazan versus Lansoprazole for the Initial Treatment of Reflux Esophagitis: A Cost-effectiveness Analysis in Japan. Intern Med 2019;58:2427-33. [Crossref] [PubMed]
- Habu Y, Hamasaki R, Maruo M, et al. Treatment strategies for reflux esophagitis including a potassium-competitive acid blocker: A cost-effectiveness analysis in Japan. J Gen Fam Med 2021;22:237-45. [Crossref] [PubMed]
- Yokoya Y, Igarashi A, Uda A, et al. Cost-utility analysis of a 'vonoprazan-first' strategy versus 'esomeprazole- or rabeprazole-first' strategy in GERD. J Gastroenterol 2019;54:1083-95. [Crossref] [PubMed]
- Briggs A, Sculpher M. An introduction to Markov modelling for economic evaluation. Pharmacoeconomics 1998;13:397-409. [Crossref] [PubMed]
- Heudebert GR, Marks R, Wilcox CM, et al. Choice of long-term strategy for the management of patients with severe esophagitis: a cost-utility analysis. Gastroenterology 1997;112:1078-86. [Crossref] [PubMed]
- Habu Y, Maeda K, Kusuda T, et al. "Proton-pump inhibitor-first" strategy versus "step-up" strategy for the acute treatment of reflux esophagitis: a cost-effectiveness analysis in Japan. J Gastroenterol 2005;40:1029-35. [Crossref] [PubMed]
- Romagnuolo J, Meier MA, Sadowski DC. Medical or surgical therapy for erosive reflux esophagitis: cost-utility analysis using a Markov model. Ann Surg 2002;236:191-202. [Crossref] [PubMed]
- Gralnek IM, Dulai GS, Fennerty MB, et al. Esomeprazole versus other proton pump inhibitors in erosive esophagitis: a meta-analysis of randomized clinical trials. Clin Gastroenterol Hepatol 2006;4:1452-8. [Crossref] [PubMed]
- He J, Ma X, Zhao Y, et al. A population-based survey of the epidemiology of symptom-defined gastroesophageal reflux disease: the Systematic Investigation of Gastrointestinal Diseases in China. BMC Gastroenterol 2010;10:94. [Crossref] [PubMed]
- Xie Z, Li H. Discussion on Discount Rate in Pharmacoeconomic Evaluation for China. Chin Health Econ 2019;38:74-7.
- Umezawa M, Kawami N, Hoshino S, et al. Efficacy of On-Demand Therapy Using 20-mg Vonoprazan for Mild Reflux Esophagitis. Digestion 2018;97:309-15. [Crossref] [PubMed]
- Miwa H, Igarashi A, Teng L, et al. Systematic review with network meta-analysis: indirect comparison of the efficacy of vonoprazan and proton-pump inhibitors for maintenance treatment of gastroesophageal reflux disease. J Gastroenterol 2019;54:718-29. [Crossref] [PubMed]
- Rantanen TK, Salo JA. Gastroesophageal reflux disease as a cause of death: analysis of fatal cases under conservative treatment. Scand J Gastroenterol 1999;34:229-33. [Crossref] [PubMed]
- Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [Crossref] [PubMed]
- Rousseau MJ, Evans JC. Key statistical assumptions and methods in one-arm meta-analyses with binary endpoints and low event rates, including a real-life example in the area of endoscopic colonic stenting. Cogent Medicine 2017;4:1334318. [Crossref]
- Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making 1993;13:322-38. [Crossref] [PubMed]
- National Bureau of Statistics of China. China Statistical Yearbook 2019. Available online: http://www.stats.gov.cn/tjsj/ndsj/2019/indexeh.htm
- Wang ZH, Yan YQ, Tong TY, et al. Estimating health-state utility values in reflux esophagitis using the EQ-5D-5L. ISPOR 2020, Orlando, FL, USA. Available online: https://www.ispor.org/heor-resources/presentations-database/presentation/intl2020-3182/100960
- Ashida K, Iwakiri K, Hiramatsu N, et al. Maintenance for healed erosive esophagitis: Phase III comparison of vonoprazan with lansoprazole. World J Gastroenterol 2018;24:1550-61. [Crossref] [PubMed]



