
Short-term and long-term outcomes of natural orifice specimen extraction surgeries (NOSES) in rectal cancer: a comparison study of NOSES and non-NOSES
Introduction
Colorectal cancer (CRC) is the third most common malignancy in both males and females in the US (1). CRC is also the second leading cause of cancer-related deaths worldwide (2). The incidence of CRC in China is also increasing (3). Rectal cancer accounts about 60–70% of all CRC. Although various therapeutic strategies have been developed during the last decades, surgery is still the most effective procedure to treat CRC. Some classical surgery types and principles have been proposed, including complete mesocolic excision (CME) for colon cancer and total mesorectal excision (TME) for rectal cancer.
Laparoscopic surgeries have also expanded the treatment options for CRC. The COLOR II study showed that laparoscopic surgery resulted in similar safety, resection margins, completeness of resection, and oncologic outcomes to that of open surgery, and recovery was improved after laparoscopic surgery. However, an incision of about 5–10 cm is still inevitable for traditional laparoscopic surgeries as well as small trocar incisions. Incision-related complications in laparoscopic CRC surgery have similar rates when compared with open surgery (4,5). Natural orifice specimen extraction surgery (NOSES), as an alternative to traditional laparoscopic and open surgery, addresses this by eliminating the need for incision. It is a well-established procedure and has been shown to result in less pain, fewer peri-operative complications, and faster recovery times (6,7). Several types of NOSES have been proposed for rectal cancer. Traditional laparoscopic surgeries (non-NOSES) have been widely conducted in clinical practice. COLOR II also showed the superiority of non-NOSES over open surgeries. By comparing with non-NOSES, the feasibility and safety of NOSES could be further verified. Also, there are relatively few studies comparing the short-term outcomes, long-term outcomes, feasibility, and safety among different types of NOSES or between NOSE surgeries and traditional laparoscopic surgeries. Here, we conducted a single-centered retrospective study to analyze the differences between different NOSES and laparoscopic surgeries for rectal cancer with the hypothesis that NOSES for rectal cancer have better short-term outcomes and long-term outcomes that are not inferior to laparoscopic and open CRC surgery. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1175/rc).
Methods
This is a retrospective comparative study. The objective of the study was to compare the short-term and long-term outcomes of NOSES and non-NOSES. Consecutive cases who underwent NOSES or non-NOSE laparoscopic rectal surgeries were identified retrospectively at a single center between 1 January 2013 and 31 December 2018 at the Second Affiliated Hospital of Harbin Medical University. The inclusion criteria were as follows: Patients with adenocarcinoma of the rectum within 15 cm of the anal verge; patients over the age of 18; and patients undergoing primary laparoscopic rectal resection; patients signed written formal consent forms; patients had complete information. The localization of the tumor was classified as the upper rectum (distal border of tumor, 10 to 15 cm from the anal verge), middle rectum (5 to 10 cm from the anal verge), or lower rectum (<5 cm from the anal verge). All operations were performed by skilled colorectal surgeons.
Medical records were retrieved from the Hospital Information System, and the following medical data were collected: demographic information (sex, age, BMI, diabetes mellitus, anemia, low albumin, and staging), diagnosis, tumor location (tumor distance from anal verge by colonoscope or MRI scan), operation time, operation duration (from skin cut to incision closure), post-operative hospital stay (the first day after operation to the day of discharge), pathological data, peri-operative complications, and medical co-morbidities (including bleeding, anastomosis leakage, incision infection, fever, ileus, unplanned reoperation, anal dysfunction, deep vein thrombosis etc.). Patients were followed-up during the inpatient period, at an outpatient clinic, or by phone call. Disease-free survival (DFS) was the length of time after primary treatment ends that the patient survives without any signs or symptoms of rectal cancer. Overall survival (OS) was defined as the time from surgery to death from rectal cancer. As many objective records were retrieved as possible to control potential bias in our study.
Operations were performed as described previously (8). NOSES for rectal cancer was categorized as specimen eversion and extra-abdominal resection (EVER), specimen extraction and extra-abdominal resection (EXER), and intra-abdominal resection and specimen extraction (IREX). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics board of the Second Affiliated Hospital of Harbin Medical University (No. GZRYS-125) and informed consent was taken from all the patients.
Statistical analysis
Quantitative data were summarized as mean ± standard deviation (SD). Statistical analysis was performed with R statistical software (R 4.1.1, R Foundation for Statistical Computing, Vienna, Austria). ANOVA test and Student’s t test were used to compare continuous data and the Dunn test was used for multiple comparison. Chi-square test or Fisher’s exact test was used to analyze categorical data. The Shapiro–Wilk test were used to test the normality of the data. “Survminer” and “Survival” packages in R were used to perform survival analysis. A two-sided P value of <0.05 was considered statistically significant. Shapiro-Wilk test was used to test the normality of continuous data.
Results
Basic characteristics comparison
A total of 439 cases were included in our study, of which 243 were traditional laparoscopic surgeries (the non-NOSES group) and 196 were NOSES. Out of the 196 NOSES cases, 78 cases underwent EVER, 66 cases underwent EXER, and 52 cases underwent IREX. Sex, BMI, age, neoadjuvant therapy, and the presence of preoperative diabetes, anemia, and low albumin were compared between the four groups (non-NOSE, EVER, EXER and IREX). The last date of follow-up was August 1, 2019. The average follow-up time was 23 months, and the total follow-up time was 7 years. No missing data existed for each case. As shown in Table 1, there was a difference in the sex distribution among the four groups; more of the cases in the non-NOSES groups that underwent traditional laparoscopic anterior resection (LAR) were male than in other groups. Further analysis found that there was no sex difference among the EVER, EXER, or IREX groups (data not shown). Interestingly, the NOSES groups had lower BMI values compared with the non-NOSES group, while no difference of BMI was observed in the NOSES groups. There was no age difference in the four groups. Analysis of pre-operative comorbidities indicated that the IREX and non-NOSES group had a higher diabetes mellitus rate when compared with the other two groups. No significant differences were observed in the anemia and low albumin rate in the four groups. Only 2 cases in the non-NOSES group received neoadjuvant chemotherapy, and no difference was shown across the four groups. There was a slight difference in the American Joint Committee on Cancer (AJCC) staging among the four groups. Although the three NOSES groups showed similar T staging distribution (P>0.05), a borderline significant difference was found across all four groups.
Table 1
| Characteristics | EVER | EXER | IREX | Non-NOSES | P value |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 40 | 26 | 24 | 145 | 0.016 |
| Female | 38 | 40 | 28 | 98 | |
| BMI (kg/m2) | 22.08 | 22.00 | 22.53 | 23.26 | 0.003 |
| Age, years (mean ± SD) | 60.62±12.03 | 59.85±9.75 | 61.36±13.27 | 60.35±11.08 | 0.901 |
| DM, n (%) | 5 (6.41) | 1 (1.51) | 10 (19.20) | 27 (11.11) | 0.002 |
| Anemia, n (%) | 1 (1.28) | 2 (3.03) | 0 | 0 | 0.055 |
| Low albumin, n (%) | 0 | 0 | 0 | 1 (0.41) | 1 |
| AJCC stage | 0.008 | ||||
| 0 | 1 | 4 | 2 | 0 | |
| I | 23 | 18 | 24 | 68 | |
| II | 24 | 26 | 12 | 99 | |
| III | 24 | 15 | 15 | 71 | |
| IV | 1 | 2 | 2 | 4 | |
| AJCC T stage | 0.036 | ||||
| T1 + T2 | 30 | 23 | 27 | 77 | |
| T3 + T4 | 41 | 38 | 25 | 165 | |
| AJCC N stage | 0.893 | ||||
| N0 | 24 | 18 | 15 | 73 | |
| N1–2 | 49 | 47 | 40 | 169 | |
| Neoadjuvant therapy, n (%) | 0 | 0 | 0 | 2 (0.82) | 1 |
EVER, specimen eversion and extra-abdominal resection; EXER, specimen extraction and extra-abdominal resection; IREX, intra-abdominal resection and specimen extraction; NOSES, natural orifice specimen extraction surgery; BMI, body mass index; SD, standard deviation; DM, diabetes mellitus; AJCC, America Joint of Cancer Committee.
Intra- and post-operative comorbidities analysis
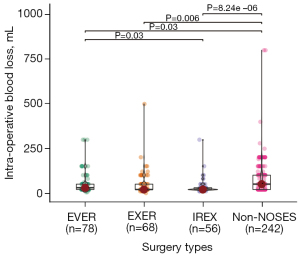
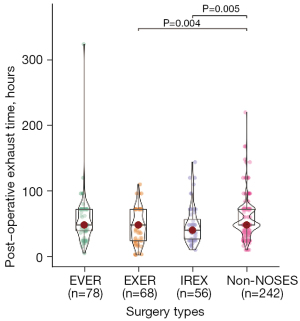
Short-term and long-term outcomes and complications were further analyzed among different groups. As shown in Table 2, there was no difference in the operation time for the four groups. Bleeding in the NOSES groups was significantly less than the non-NOSES group, while the EVER, EXER, and IREX groups had similar blood loss (Figure 1). The EXER and IREX groups showed less time to first post-operative flatus than the non-NOSES group, while no difference was observed among NOSES groups (Table 2; Figure 2). The same lymph nodes were harvested for all four groups. There was no difference in the post-operative stay, anastomosis bleeding, intestinal obstruction, unplanned reoperation, anal dysfunction, or deep vein thrombosis among all cases. The tumor diameter was similar among the three NOSES groups. However, more protective ileostomies were performed for the non-NOSES group.
Table 2
| Short-term outcome | EVER | EXER | IREX | Non-NOSES | P value |
|---|---|---|---|---|---|
| Operation time (minute, mean ± SD) | 190±47.06 | 190±63.62 | 183±31.83 | 186±50.74 | 0.833 |
| Intra-operative blood loss (mL, mean ± SD) | 57.05±62.78 | 52.65±68.19 | 36.52±43.99 | 76.12±90.11 | 0.002 |
| Number of total LNs (mean ± SD) | 13.01±5.71 | 13.17±5.67 | 13.55±5.71 | 13.68±5.32 | 0.818 |
| Post-operative exhaust time (hour, mean ± SD) | 54.68±37.80 | 45.06±24.69 | 47.91±28.93 | 56.94±27.69 | 0.012 |
| Post-operative hospital stay (day, mean ± SD) | 14.14±7.07 | 12.94±5.89 | 12.50±4.28 | 14.15±6.70 | 0.216 |
| Anastomosis bleeding, n (%) | 0 | 0 | 1 (1.92) | 2 (0.82) | 0.529 |
| Anastomosis leakage, n (%) | 4 (5.12) | 3 (4.55) | 1(1.92) | 4 (1.64) | 0.207 |
| Intestinal obstruction, n (%) | 0 | 0 | 0 | 3 (1.23) | 1 |
| Unplanned reoperation, n (%) | 0 | 0 | 1 (1.92) | 4 (1.64) | 0.562 |
| Anal dysfunction, n (%) | 4 (5.12) | 5 (7.57) | 4 (7.69) | 19 (7.82) | 0.893 |
| Deep vein thrombosis, n (%) | 0 | 0 | 0 | 1 (0.41) | 1 |
| Tumor longest diameter (cm, mean ± SD) | 3.48±1.59 | 3.67±1.60 | 3.68±1.59 | 0.492 | |
| Protective ileostomy | 13 | 3 | 1 | 37 | 0.003 |
EVER, specimen eversion and extra-abdominal resection; EXER, specimen extraction and extra-abdominal resection; IREX, intra-abdominal resection and specimen extraction; NOSES, natural orifice specimen extraction; SD, standard deviation.
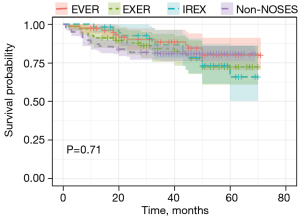
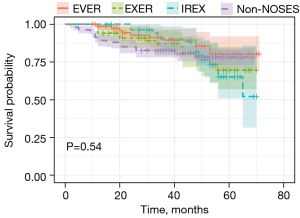
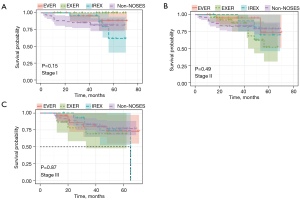
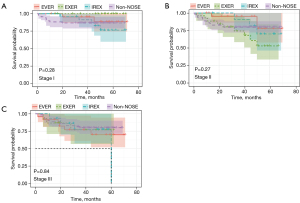
DFS and OS analyses were also conducted to assess the oncologic outcome of NOSES. As shown in Figure 3 and Figure 4, neither DFS nor OS showed differences among the three NOSES groups or among all four groups. Survival analysis was also performed in different AJCC stages, and no difference was observed in all groups (Figures 5,6).
Discussion
NOSES to treat CRC has been widely accepted since it was systematically proposed in 2013, especially during the past 10 years. Research has shown that NOSES have various advantages. Without abdominal incision, pain will be reduced, and enhanced recovery will be achieved. NOSES also brings cosmetic and psychological benefits to CRC patients. Rectal cancer accounts for 40–60% of all CRC, and evaluation is essential to compare the short-term and long-term outcomes of NOSES for rectal cancer with traditional laparoscopic surgeries or within NOSES groups. Our results showed that there were no differences between NOSE and traditional laparoscopic surgeries. Result also showed there were no differences among different types of NOSES in rectal cancer.
Interestingly, NOSES were found to be have less blood loss than traditional non-NOSES group. This might be associated with the smaller BMI and earlier T staging of patients in the NOSES groups. Previous studies showed that operative bleeding was significantly increased when comparing normal-weight patients to overweight patients (9,10). Advanced T stage is significantly related to larger tumor size (11), and patients with large sized tumors have more blood loss in CRC surgery (12). Data also correlated blood loss with short-term and long-term outcomes of CRC. The amount of intraoperative blood loss was associated with significant differences in the OS and DFS of patients with stage II/III CRC who received curative resection (13). Another study also found that the degree of blood loss during surgery for colon cancer was a factor that influences long-term survival (14). Furthermore, blood loss during surgery increased the risk of subsequent surgery for small bowel obstruction (15). However, more studies are needed to compare the intraoperative blood loss between NOSE and non-NOSE surgeries in CRC and for various T stages and tumor sizes.
There was no difference in the operation time between the NOSES group and the non-NOSE group, which is inconsistent with other studies (16,17). We speculate that the difference was mainly due to the proficiency of these surgeries. NOSES have been proposed and widely applied since 2013, and the surgeons in our center quickly attained maximal performance in the execution of NOSES. Our results indicate that NOSES are replicable and feasible. There was a difference in the age distribution between the NOSES and non-NOSES groups.
We also found a shorter post-operative gas exhaust time in NOSE compared with non-NOSE surgeries, which is consistent with previous studies (17,18). This result further confirmed the advantages of NOSES to significantly shorten the postoperative gas exhaust time without increasing other complications. This observation was closely associated with post-operative pain. Research has shown that NOSES reduce patients’ postoperative pain by avoiding an abdominal extraction site (19,20). This relief of postoperative pain therefore contributed to the rapid rehabilitation of patients, including a shortened post-operative gas exhaust time (21). Further, more diverting ileostomies were created in non-NOSE rectal cancer surgeries when compared with NOSE surgeries, while there was no difference between the anastomosis leakage. This data indicated that ileostomy might have a limited role in decreasing the anastomosis leakage. However, more detailed studies are needed to further verify this conclusion.
There was no difference in the long-term prognosis between NOSES and non-NOSES groups. The 5-year OS and DFS showed that patients receiving NOSES had a comparable prognosis with those receiving non-NOSES. This result demonstrates that NOSES in rectal cancer are oncologically safe.
This study has some limitations. First, it is a retrospective study. Some key statistics cannot be recorded and analyzed, and significant biases may affect the selection of controls. Some prospective studies are ongoing, and more reliable data will be reported. Second, the number of included cases is small, which makes it difficult to determine if a particular outcome is a true finding. Third, we only included patients who received rectal cancer resection, and it is unknown if the conclusion applies to colon cancer.
In conclusion, our study examined the short-term and long-term outcome of NOSES in rectal cancer and demonstrated that NOSES, at least in rectal cancer, are safe and feasible treatment options. These results warrant further study, and NOSES should be implemented in the treatment of CRC.
Acknowledgments
Funding: This research was funded by Zhejiang Province Medical Health Science and Technology Project (Grant No.2022498823).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1175/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-1175/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-1175/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of the Second Affiliated Hospital of Harbin Medical University (No. GZRYS-125) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Xu R, Wang W, Zhu B, et al. Disease characteristics and treatment patterns of Chinese patients with metastatic colorectal cancer: a retrospective study using medical records from China. BMC Cancer 2020;20:131. [Crossref] [PubMed]
- Hackert T, Uhl W, Büchler MW. Specimen retrieval in laparoscopic colon surgery. Dig Surg 2002;19:502-6. [Crossref] [PubMed]
- Kuhry E, Schwenk WF, Gaupset R, et al. Long-term results of laparoscopic colorectal cancer resection. Cochrane Database Syst Rev 2008;CD003432. [Crossref] [PubMed]
- Kang J, Min BS, Hur H, et al. Transanal specimen extraction in robotic rectal cancer surgery. Br J Surg 2012;99:133-6. [Crossref] [PubMed]
- Wolthuis AM, Meuleman C, Tomassetti C, et al. How do patients score cosmesis after laparoscopic natural orifice specimen extraction colectomy? Colorectal Dis 2015;17:536-41. [Crossref] [PubMed]
- Guan X, Liu Z, Longo A, et al. International consensus on natural orifice specimen extraction surgery (NOSES) for colorectal cancer. Gastroenterol Rep (Oxf) 2019;7:24-31. [Crossref] [PubMed]
- Hede P, Sörensson MÅ, Polleryd P, et al. Influence of BMI on short-term surgical outcome after colorectal cancer surgery: a study based on the Swedish national quality registry. Int J Colorectal Dis 2015;30:1201-7. [Crossref] [PubMed]
- Ogunsua AA, Touray S, Lui JK, et al. Body mass index predicts major bleeding risks in patients on warfarin. J Thromb Thrombolysis 2015;40:494-8. [Crossref] [PubMed]
- Li X, An B, Ma J, et al. Prognostic Value of the Tumor Size in Resectable Colorectal Cancer with Different Primary Locations: A Retrospective Study with the Propensity Score Matching. J Cancer 2019;10:313-22. [Crossref] [PubMed]
- Jiang W, Fang YJ, Wu XJ, et al. Intraoperative blood loss independently predicts survival and recurrence after resection of colorectal cancer liver metastasis. PLoS One 2013;8:e76125. [Crossref] [PubMed]
- Tamagawa H, Numata M, Aoyama T, et al. Impact of Intraoperative Blood Loss on the Survival of Patients With Stage II/III Colorectal Cancer: A Multicenter Retrospective Study. In Vivo 2021;35:3483-8. [Crossref] [PubMed]
- Mörner ME, Gunnarsson U, Jestin P, et al. The importance of blood loss during colon cancer surgery for long-term survival: an epidemiological study based on a population based register. Ann Surg 2012;255:1126-8. [Crossref] [PubMed]
- Egenvall M, Mörner M, Påhlman L, et al. Degree of blood loss during surgery for rectal cancer: a population-based epidemiologic study of surgical complications and survival. Colorectal Dis 2014;16:696-702. [Crossref] [PubMed]
- Zhou ZQ, Wang K, Du T, et al. Transrectal Natural Orifice Specimen Extraction (NOSE) With Oncological Safety: A Prospective and Randomized Trial. J Surg Res 2020;254:16-22. [Crossref] [PubMed]
- Zhou Z, Chen L, Liu J, et al. Laparoscopic Natural Orifice Specimen Extraction Surgery versus Conventional Surgery in Colorectal Cancer: A Meta-Analysis of Randomized Controlled Trials. Gastroenterol Res Pract 2022;2022:6661651. [Crossref] [PubMed]
- Ding Y, Li Z, Gao H, et al. Comparison of efficacy between natural orifice specimen extraction without abdominal incision and conventional laparoscopic surgery in the treatment of sigmoid colon cancer and upper rectal cancer. J BUON 2019;24:1817-23. [PubMed]
- Pedrazzani C, Menestrina N, Moro M, et al. Local wound infiltration plus transversus abdominis plane (TAP) block versus local wound infiltration in laparoscopic colorectal surgery and ERAS program. Surg Endosc 2016;30:5117-25. [Crossref] [PubMed]
- Zhu Z, Wang KJ, Orangio GR, et al. Clinical efficacy and quality of life after transrectal natural orifice specimen extraction for the treatment of middle and upper rectal cancer. J Gastrointest Oncol 2020;11:260-8. [Crossref] [PubMed]
- Yamamoto M, Asakuma M, Tanaka K, et al. Clinical impact of single-incision laparoscopic right hemicolectomy with intracorporeal resection for advanced colon cancer: propensity score matching analysis. Surg Endosc 2019;33:3616-22. [Crossref] [PubMed]


