Importance of tumor infiltrating lymphocytes in non-small cell lung cancer?
Lung cancer, of which non-small cell lung cancer (NSCLC; 85%) is the largest subgroup, constitute our most deadly malignant disease with a 5-year survival of merely 15% (1). This malignancy, as well as other solid neoplasms, is not simply an accumulation of malignant cells, but represents also a microenvironment containing endothelial cells, fibroblasts, structural components and infiltrating immune cells. This microenvironment highly influences tumor development, invasion, metastasis and patient outcome.
During the last 15 years, groundbreaking adaptive immunity studies by Schreiber and coworkers proved the existence of cancer immunosurveillance in the microenvironments of neoplasms (2-5), which has been further refined into cancer immunoediting, incorporating a broader view of the tumor-immune system interactions (6). During this period there has been a dramatic change of view on malignancy, from being a considered autonomous cellular disease comprising six biological capabilities (7), to a regulated disease involving the immune components of its microenvironment (8).
The research interest concerning immune cell infiltration in cancers has been rapidly increasing. During the period 2000–2015, there were 17 larger clinical studies (>200 cases) on the role of tumor-infiltrating immune cells. Of these, as many as 13 (76%) have been published since 2011 (9), illustrating the growing interest in this research topic. In near all these studies (15 of 17), immune cells were identified by specific cluster designation (CD) markers, following immunohistochemical (IHC) staining of the slides. Cytotoxic T (CD8+) cells were consistently associated with a beneficial prognostic impact in all studies based on the CD8 and/or CD3 markers (9,10). CD8+ T cells constitute 80% of CD3+ cells. In half of these studies, the positive prognostic impact was striking and independent of other prognostic factors.
The study by Brambilla and coworkers, recently published in Journal of Clinical Oncology (11), investigated the role of tumor lymphocytic infiltrations (TILs) in NSCLC. This was undertaken in a training set (one trial, n=783) and a validation set (three trials, n=763), which originally evaluated the benefit of platinum-based adjuvant chemotherapy in NSCLC. The object in this study was to assess whether TILs may have prognostic and/or predictive impact in NSCLC. Median follow-up was 4.8 and 6.0 years respectively in training and validation set, respectively. Two experienced lung tumor pathologists scored TLI on hematoxylin-eosin stained whole slides from resected NSCLC tumors, not applying IHC-staining for CD markers. The intensity of lymphocytes was scored according to four categories (minimal, mild, moderate and intense). Eventually a binary scoring system was used, in which the first three categories were merged and defined as low intensity group. The authors found the TLI to be intense in a minority (11%) of patients in the discovery set, and even significantly less frequent (6%) in the validation set (P<0.001). In their study cohorts, intense TLI led to significantly prolonged overall survival (OS), disease-free survival (DFS) and specific DFS (SDFS) in the discovery set (OS: HR, 0.56, 95% CI, 0.38–0.81, P=0.002; DFS: HR, 0.59, 95% CI 0.42–0.83, P=0.002; SDFS: HR, 0.56, 95% CI, 0.38–0.82, P=0.003) which was confirmed in the validation set (OS: HR, 0.45, 95% CI, 0.23–0.85, P=0.01; DFS: HR, 0.44, 95% CI 0.24–0.78, P=0.005; SDFS: HR, 0.42, 95% CI, 0.22–0.80, P=0.008). When data from the training and validation set were pooled, the HR was 0.53. However, no significant predictive effects were observed for TLI.
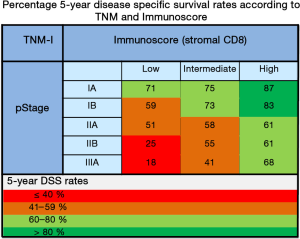
The authors should be commended for carrying out the hitherto largest clinical study (n=1,546) on the prognostic relevance of T lymphocyte infiltration in NSCLC tumors, including both training and validation cohorts. Only two of the previously published similar studies included validation cohorts (12,13). In the study by Donnem et al., one training set and three external validation sets with a total of 797 patients were examined (12). In this study, density of CD8+ T cells was assessed only in stroma as our group previously found stromal CD8+ density (14), rather than within the epithelial cancer cell nests to have significantly stronger prognostic impact with respect to DFS, disease-specific survival (DSS), and OS. Subgroup analyses revealed an independent significant prognostic impact of stromal CD8+ TIL density within each examined NSCLC pathologic stage (pStage; IA, IB, IIA, IIB, and IIIA; Figure 1). The prognostic impact of TIL density equaled that of NSCLC pStage in the multivariate analysis (12).
In the study by Schalper and coworkers (13), 552 NSCLC patients (two cohorts, n=202 and 350) were examined with respect to levels of CD3+, CD8+, and CD20+ TILs in resected tumor specimens. The density of CD8+ and CD3+ cells were statistically significantly associated with prolonged survival in both cohorts. However, only CD8+ TILs were an independent prognosticator in the multivariate analysis.
In a recently published meta-analysis of 29 small and large studies on the prognostic role of various TILs in NSCLC (15), data strongly document that high densities of tumor-infiltrating cytotoxic CD8+ and CD3+ T cells, assessed in the stromal or tumor epithelial compartment of NSCLC, are associated with significant beneficial patient outcomes mediated by immunity towards the tumor. But it is emphasized that stromal assessments of TILs appear to have a superior prognostic impact when compared with epithelial assessments, which is somewhat in agreement with our previous studies (14) and Brambilla and coworkers (11).
High density of TILs, preferably CD8+ TILs, should be a good candidate marker for establishing a TNM immunoscore. In Norway, there is a national initiative for a prospective study validating an immunoscore for NSCLC (16), comparable to the French initiative in colorectal cancer (17). Though, there are still several challenges to be sorted out before establishing an immunoscore. These are among others (I) IHC staining procedures; (II) scoring system; and (III) which cellular compartment to score.
An established standardized histopathological assessment, such as immunoscore, added to the TNM classification (TNM-I) may be essential in launching a highly improved prognostic, if not also a predictive tool. A TNM-I for lung cancer will need to be evaluated and validated in large prospective studies prior to implementation. The TNM classification for lung cancer is presently under revision, to what will appear as the 8th edition (18). The immune contexture will in the future have to be evaluated for incorporation into future editions of the TNM classifications, as research has demonstrated substantial room for improvements regarding prognostic and possibly also predictive value. As outlined above, Immunoscore may delineate significant variety in NSCLC survival within each pStage. A score involving assessment of in situ immunity will also be fundamental for optimization of immunotherapy and immunomodulation.
Acknowledgements
We are grateful to the Norwegian Cancer Society and Northern Norway Health Region Authority for financial support.
Footnote
Provenance: This is a Guest Editorial commissioned by Section Editor Jianrong Zhang, MD (Department of Thoracic Surgery, First Affiliated Hospital of Guangzhou Medical University, Guangzhou Institute of Respiratory Disease, Guangzhou, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [Crossref] [PubMed]
- Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol 2004;22:329-60. [Crossref] [PubMed]
- Koebel CM, Vermi W, Swann JB, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature 2007;450:903-7. [Crossref] [PubMed]
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011;331:1565-70. [Crossref] [PubMed]
- Shankaran V, Ikeda H, Bruce AT, et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 2001;410:1107-11. [Crossref] [PubMed]
- Mittal D, Gubin MM, Schreiber RD, et al. New insights into cancer immunoediting and its three component phases--elimination, equilibrium and escape. Curr Opin Immunol 2014;27:16-25. [Crossref] [PubMed]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100:57-70. [Crossref] [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Bremnes RM, Busund LT, Kilvær TL, et al. The Role of Tumor-Infiltrating Lymphocytes in Development, Progression, and Prognosis of Non-Small Cell Lung Cancer. J Thorac Oncol 2016. pii: S1556-0864(16)00352-X.
- Gooden MJ, de Bock GH, Leffers N, et al. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer 2011;105:93-103. [Crossref] [PubMed]
- Brambilla E, Le Teuff G, Marguet S, et al. Prognostic Effect of Tumor Lymphocytic Infiltration in Resectable Non-Small-Cell Lung Cancer. J Clin Oncol 2016.JCO630970. [Epub ahead of print]. [PubMed]
- Donnem T, Hald SM, Paulsen EE, et al. Stromal CD8+ T-cell Density—A Promising Supplement to TNM Staging in Non-Small Cell Lung Cancer. Clin Cancer Res 2015;21:2635-43. [Crossref] [PubMed]
- Schalper KA, Brown J, Carvajal-Hausdorf D, et al. Objective measurement and clinical significance of TILs in non-small cell lung cancer. J Natl Cancer Inst 2015;107:dju435. [Crossref] [PubMed]
- Al-Shibli KI, Donnem T, Al-Saad S, et al. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin Cancer Res 2008;14:5220-7. [Crossref] [PubMed]
- Geng Y, Shao Y, He W, et al. Prognostic Role of Tumor-Infiltrating Lymphocytes in Lung Cancer: a Meta-Analysis. Cell Physiol Biochem 2015;37:1560-71. [Crossref] [PubMed]
- Donnem T, Kilvaer TK, Andersen S, et al. Strategies for clinical implementation of TNM-Immunoscore in resected nonsmall-cell lung cancer. Ann Oncol 2016;27:225-32. [Crossref] [PubMed]
- Galon J, Mlecnik B, Bindea G, et al. Towards the introduction of the 'Immunoscore' in the classification of malignant tumours. J Pathol 2014;232:199-209. [Crossref] [PubMed]
- Rami-Porta R, Bolejack V, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for the Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2015;10:990-1003.


