
Peripheral muscle force balance/imbalance in neurological binary control of bladder function and dysfunction
Highlight box
Key findings
• Bladder control is binary, balanced, with cortical and peripheral components.
What is known and what is new?
• There is no concept of peripheral control of the bladder.
• Balanced peripheral control of the bladder. Pelvic muscle forces contract against ligaments to: (I) close the urethra; (II) open it; (III) stretch the vagina to prevent urge incontinence.
What is the implication and what should we change now?
• Ligament damage weakens muscles contracting against them to unbalance the system and cause dysfunctions I, II, III above.
• A change in thinking that acknowledges that dysfunctions of I, II, III above, are potentially curable by repairing the appropriate pelvic ligaments.
Introduction
To me it has always been obvious that in general the reason behind female urinary incontinence has to be looked for outside the bladder, i.e., in the structures supporting the urethra and bladder neck—specifically ligaments, pelvic floor muscles and vagina. If symptoms of urinary incontinence arise from a dysfunctional anatomy in the aforementioned structures, then function should come with restoration of anatomy.”—Professor Axel Ingelman-Sundberg, Karolinska Insitutet, 1990.
1990 Integral Theory URL: https://obgyn.onlinelibrary.wiley.com/toc/16000412/1990/69/S153
The pathogenesis of urge incontinence, even today, is said to be unknown. The 1976 International Continence Society (ICS) definitions brought some order into the chaos existing previously for stress and urge incontinence. Prior to this, urge was variously called “active incontinence”, “uninhibited” or “hypertonic bladder”, “unstable bladder”.
That the control of the bladder is neurological, is not arguable. The question is how? The early physiologists related the sensations of bladder filling to the afferent nerves running from the bladder to the spinal cord and the efferent nerves running from the spinal cord to the bladder. Barrington described a series of reflexes for micturition (1). Feneley and Harrison described a plethora of initiatory and inhibitory centres in the cerebral cortex, pons, medulla, cerebellum, midbrain, basal ganglia and hypothalamus (2).
A short historical view
Historically, the role of neurological circuits in bladder function, OAB and other bladder conditions has been confused and unclear. Fearnsides described “complete and incomplete interferences” with the power of holding urine both on the “effector side and on the afferent side” (3). During cystometric studies carried out by Parker and Rose (4), characteristic patterns similar to those found with neurological lesions were observed. Lapides et al. described the “uninhibited neurogenic bladder” in a group of patients who had symptoms of urgency, frequency and precipitate micturition, but no evidence of neurological disturbance (5). Lapides et al. attributed such disturbances to incomplete development of cerebral integration much as occurs in a young child. These patterns were described as “dyssynergic detrusor dysfunction” by Hodgkinson et al. (6), “psychogenic bladder” by Youssef (7), and “uninhibited bladder” by Ingelman-Sundberg (8). These studies introduced concepts that the cause of urge incontinence could be neurological or even psychological, some of which have currency today.
Cortical control of the bladder was described by Griffiths in 2004 (9). “Symptoms such as overactive bladder (OAB) represent disorders of bladder control. Functional brain scanning by positron emission tomography (PET) and functional magnetic resonance imaging (MRI) suggest that normal control is exerted by a network of regions in the emotional nervous system, including periaqueductal gray, thalamus, insula, anterior cingulate, and prefrontal cortex.”
The question with such scans is, “What are they measuring?” Griffiths regards “OAB” (urgency) solely as caused “by a network of regions in the emotional nervous system” (9). However, a purely cortical explanation as posited by Griffiths, cannot explain cure of OAB symptoms by ligament repair which was reported by several surgical studies in many hundreds of women (10-18). The Integral Theory Paradigm (ITP) interprets “lighting up” of areas of the brain detected by cortical scans as excess afferent impulses from the stretch receptors “N”, in spite of all attempts to suppress them by the control systems, cortical (Figure 1, white arrow) and peripheral (Figure 1, large red arrows).
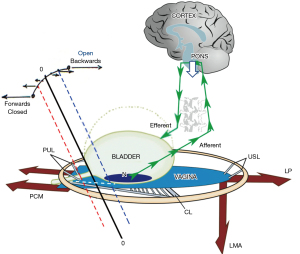
Bladder control is binary, from outside the bladder
Feedback control exists in all natural systems and can be expressed by simple mathematical formulae (19). The unstable bladder by definition has difficulty in maintaining the closed state during filling and so swings inappropriately into the open state.
In 1993, it was urodynamically demonstrated, that the bladder was a prematurely activated micturition reflex (20) regulated by a binary feedback control system, with two anatomical components, cortical and peripheral (musculo/ligamentous) (21). Closure was the dominant state. When the bladder was full, and the woman was ready to micturate, the “closure” state reflexly shut down and the “open” state dominated, emptying by an activated micturition reflex.
Cortical binary control
The central facilitatory and inhibitory systems, present in all parts of the brain (2), act as Boolean gatekeepers, opening or closing the gates (Figure 2) for the neurological impulses, as they pass up and down the central nervous system, depending on whether the micturition system is in the open or closed state.
Peripheral binary control
In a normal woman, a sensitive peripheral musculoelastic neurological control mechanism (Figure 1), maintains continence: as the bladder fills, stretch and volume receptors “N” are stimulated, and the urethral pressure reflexly rises due to a simultaneous “extrinsic muscle contraction” (Figure 1, large arrows) (22). The three reflex, cortically controlled, opposite pelvic muscle forces act in a balanced way, with zero resultant force at the bladder neck (10). See p.20, Acta URL: https://obgyn.onlinelibrary.wiley.com/toc/16000412/1990/69/S153 (also, see Video S1).
Adequate elasticity is required in the bladder neck area of the vagina “zone of critical elasticity” (ZCE), to allow the separate actions of the opposite muscle forces. Any scarring at ZCE may link these forces to unbalance the system to cause massive urine loss (10) (see Acta URL pp.8,12,14,15,16,19,21).
Surgical correction of the peripheral control system
An estimated 10 million midurethral sling (MUS) operations between 1996 and 2018 by repair of the pubourethral ligaments (PULs), cure of OAB symptoms (urge, frequency, nocturia) by uterosacral ligament (USL) repair (15-18), substantially support the 1990 core Integral Theory discovery, that control of continence and micturition was not from the bladder itself, but from outside the bladder, mainly from pelvic muscles contracting against competent suspensory ligaments (Figure 1) (10). The Integral Theory stated that loose, collagen-deficient ligaments caused prolapse and bladder dysfunctions of: closure [stress urinary incontinence (SUI)]; evacuation (retention); deficient micturition reflex control (OAB) (10). These statements were validated surgically by cure of SUI (11,12), and intrinsic sphincter defect (13) with a MUS (11-13). Cure of the following conditions with a USL sling further validated the Integral Theory predictions: OAB (frequency, urge, nocturia) (14,15), interstitial cystitis/bladder pain syndrome (16), 3rd and 4th degree prolapse (17), urinary retention and “underactive bladder” (UAB) (18).
Normal balanced urethral closure (continence)
For urge control, the PONS (white downward arrows) directly blocks afferent impulses from the urothelial stretch receptors “N” (Figure 1). In addition, cortical efferent impulses activate both oppositely acting pelvic muscle forces equally (large arrows): pubococcygeus muscle (PCM) levator plate (LP), conjoint longitudinal muscle of the anus (LMA). The muscles stretch the vaginal membrane equally, like a trampoline, to support “N” from below, with zero resultant force (0-0) at the bladder neck (see Video S1). For control of urine loss on coughing, the brain senses the cough coming and sends a signal to the peripheral mechanism to close the urethra 0.25 seconds prior to coughing being recorded, to increase the urethral pressure (2). The balance of forces alters, and 0-0, in Figure 1, moves forward to the red broken line to close the urethra, elevating urethral pressure.
Normal unbalancing of urethral opening (micturition)
Normal unbalancing of the system happens only during micturition, when the bladder is full and needs to evacuate, (Figure 1). As illustrated in Figure 1, the hydrostatic pressure of the urine presses on the urothelial stretch receptors “N” to activate afferent impulses to the cortex from “N”. At a critical point, the micturition reflex is activated, and the woman feels an “urge to go”. If convenient to empty, the cortex gives a signal for the binary system to go into “open” phase and the equilibrium point 0-0 moves backwards towards the vertical broken blue lines. Reflex cortical inhibition (white arrow) is released. Peripherally, the forward vector (PCM) relaxes, the backward vectors (LP/LMA) stretch the vagina backwards and downwards to open out the posterior urethral wall (white broken lines below the urethra) in preparation for the detrusor contraction which empties the bladder (10).
If inconvenient to empty
If inconvenient to empty, the cortex gives a signal for the system to go into “closed” phase, indicated by the broken red lines in Figure 1. Cortical inhibition (white arrow) suppresses the afferent impulses from “N”. Efferent impulses go out to the oppositely-acting pelvic muscles indicated by the large arrows in Figure 1 to close the urethra and to stretch the vagina like a trampoline to prevent afferent signals from “N”, inappropriately activating the opening (micturition) reflex.
Dysfunctional unbalancing of muscle forces
Simplistically, weak PULs unbalance the binary system so its balance moves to the blue line, “open” (incontinence) mode (Figure 1). With weak USLs, the balance moves to the red line, “closed” (retention) mode. According to the Integral Theory (10) weak or lax ligaments are the main cause of imbalance in the binary system. With reference to Figure 1, (simplistically) the PCM contracts against the PUL; the LP/LMA contract against the USL. Weak PULs unbalance the binary system so its balance moves to the blue line, “open” mode (stress incontinence) and also urge as well, if the PCM cannot stretch the vagina sufficiently forward to support the stretch receptors “N”. With weak USLs, if PUL and PCM are sound, the balance moves to the red line, “closed” mode to cause the urinary retention characteristic of Fowler’s syndrome and UAB. The same USL weakness may prevent the LP/LMA from stretching the vagina backwards to support “N”, so “N” fires off at a low bladder volume to activate micturition prematurely [OAB, detrusor overactivity (DO)], explaining how OAB and UAB can co-exist.
OAB and DO
In 1993, it was urodynamically demonstrated that OAB and DO were identical to a prematurely activated, but otherwise normal micturition (20). With reference to Figure 1, based on the binary system of control (21), what is now termed as OAB (urge, frequency, nocturia) or “urodynamic detrusor instability” (now DO) is a consequence of an anatomical defect in some part of the feedback control system (Figure 1). The anatomical defect, whether it was central or peripheral, unbalanced the system, so it swung between the “open” and “closed” states (21). This unstable state was called “detrusor instability” in the past, now “OAB”. With reference to the binary system (Figure 1), weak PULs or USLs weaken the muscle forces which stretch the vagina to support the stretch receptors “N”; “N” fires off afferent impulses which activate the micturition reflex; the “open” mode temporarily dominates, and this is experienced as “urge”. When the closure reflex regains control, the micturition reflex is suppressed, and the urge subsides.
A low compliance pattern
A low compliance pattern on cystometric testing indicates that the micturition reflex has been activated but is being successfully suppressed by the cortex (21) (Figure 1, downward white arrows). It has been urodynamically demonstrated that a handwashing test1 can release the cortical inhibition, so the equilibrium point 0-0 of the system is pushed into “open phase” (Figure 1, broken blue lines), resulting in detrusor contraction and urine loss (20,21).
Unbalanced mechanical closure (stress urinary incontinence)
During urodynamic testing, the patient is asked to cough. 0.25 seconds before the cough is registered (23), the cortex sends a signal to the peripheral control mechanism where the closure reflex is activated. With reference to Figure 1, the forward-acting PCM closes the distal urethra (10). If PULs are weak, so is the force of PCM which contracts against it. The balance of muscle forces is lost; 0-0 moves backwards to “open” phase (Figure 1, blue broken lines). As a consequence, LP/LMA pulls the bladder base backwards/downwards and the urethra is opened out (Figure 1, white broken lines below the urethra); the urethral resistance to flow is exponentially lowered (Poiseuille’s Law) and urine runs out on coughing.
“Mixed” incontinence
Up to 70–80% of women with SUI have “mixed” incontinence, stress and urge. A specific causation for urge is added to that for SUI: tensioning of the vagina below “N”, requires equal muscle force contributions from both the PCM and LP/LMA (“trampoline effect”) (Figure 1). The same loose PULs which cause SUI, weaken the forward vector, PCM, which contracts forwards against it; the vagina cannot be stretched sufficiently to support “N” from below; consequently, “N” may fire off afferent impulses to cause urge in addition to the SUI.
Unbalanced mechanical opening (urinary retention)
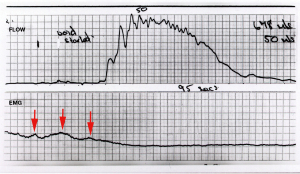
Unbalanced mechanical opening can result in symptoms of UAB or urinary retention (18). With reference to Figure 1, when ready to micturate, the cortex signals the system to move from 0-0 stable closed to “open” phase (broken blue lines), the PCM relaxes, the LP/LMA open out the urethral outflow tube; the electromyography (EMG) and flow chart are normal (Figure 2). If the USL are weak, the backward vector forces, the LP/LMA, which contract against the USL weaken and cannot adequately open the posterior urtethral wall. The detrusor has to contract against a (relatively) unopened urethra and is perceived by the woman as “obstructed micturition”, with a typical EMG and flow chart (Figure 3).
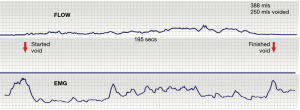
With reference to Figure 1, Fowlers’ syndrome (24) and UAB are explained as follows (18): USLs are congenitally weak, which weakens LP/LMA which contract against them. The (relatively) stronger slow-twitch forward muscles (PCM) pull the vagina and equilibrium point 0-0 forwards to the red broken lines. This imbalance of forces overtensions the distal vagina and closes the distal urethra more tightly as reported in Fowler’s syndrome (24), so that the forward stretching creates a firmer support below the urothelial stretch receptors “N”. In consequence of the firmer base created below “N”, a greater hydrostatic pressure, (a higher bladder volume) is needed to activate the afferent impulses from “N” so as to initiate the micturition reflex. This explains the greater resting bladder volumes, retention, slow emptying times reported in UAB and Fowler’s syndrome (25).
Urinary urge or retention from abnormalities in the binary neurological circuit
In the normal neurological circuit, illustrated in Figure 1, the afferent nerves from “N” are activated by hydrostatic bladder pressure to transmit impulses to the brain. The efferent nerves transmit impulses from the cortex which instruct the peripheral musculo-ligamentous control system to close the urethra, open it, or stretch the vagina to support “N” and prevent premature activation of the micturition reflex, which is sensed as “urge”.
Stimulation of urothelial stretch receptors “N”, by a local lesion, be it a cervical fibroid pressing on the bladder, base, bladder infection, urothelial cancer, can send excessive afferent impulses to the cortex (Figure 1, small green arrows), which are interpreted as urge. Once a critical mass is attained, the impulses activate the micturition reflex to cause the bladder to empty (urge incontinence). Once activated, the mechanics of micturition take over: the PCM relaxes, which allows the LP/LMA to open out the urethra “funnelling” (Figure 1, white broken lines), and the detrusor contracts to empty.
Blocked cortical inhibition be it from vascular (stroke), tumour, or multiple sclerosis (MS), may allow unhindered passage of afferent impulses to the micturition centre to activate micturition, causing uncontrolled urine leakage. Damaged efferent nerves, for example, by MS, may prevent activation of the peripheral control mechanism and also result in constant urine leakage. Prevention of afferent stimulation from “N” (Figure 1), by MS or spinal cord transection, may result in urinary retention.
Urodynamic manifestations of the binary system with urge incontinence
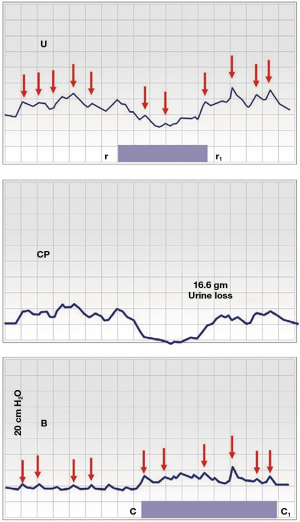
Figure 4 is a cystometric urodynamic graph. The phasic pattern of urodynamic “DO” can be explained as a battle for dominance by the two modes of the binary system, “closed” (Figure 1, red line) and “open” (Figure 1, blue line). Once the micturition reflex (DO pattern) is activated by the brain, the proximal urethral closure pressure (CP) falls (from r to r1), and, some seconds later, in “B”, the bladder begins to contract (from c to c1) (20,25). Forced fast filling of the bladder (100 mL/minute) sends rapid afferent impulses from “N” to the brain and the closure reflex is activated; the forward vector, PCM, contracts forwards repeatedly to close the distal urethra (Figure 4, small red arrows). Meanwhile the micturition reflex is being continuously stimulated by rapid afferent impulses from “N” (Figure 1). At “r”, the “open” binary mode became temporarily dominant; the balance of forces switched from “closed” to “open” and 16.6 gm urine was lost ; at r1, the “closed” mode regained dominance, CP increased. The phasic urodynamic pattern in Figure 4, “U”, “CP”, which characterises “DO”, can be anatomically explained as follows: there is a 3–8 second delay in the afferent/efferent feedback control system (Figure 1) between the low pressure, where the micturition reflex is dominating, and high pressure, where the closure reflex is dominating (21). The time interval taken to switch from “closed” mode to “open” mode is expressed as a phasic pattern on the recording graphs, “U” and “CP”, in Figure 4. The pressure peaks (red arrows) which are active throughout, indicate repeated contractions by the pelvic striated muscle to close the urethra (under direction from the closure reflex).
Urinary retention from neurological circuit abnormalities
Prevention of afferent signals from stretch receptors “N” reaching the cortex, for example, by MS lesions in the afferent pathway from “N” to the micturition centre, may prevent activation of micturition to cause urinary retention. The detrusor has to contract against an unopened urethral tube. The balance of forces moves permanently forward to “closed” (Figure 1, red line). Spinal cord transection will sever both afferent and efferent pathways (Figure 1, green arrows) to cause urinary retention, at least initially.
Massive urine loss from a scarred bladder neck, tethered vagina syndrome (TVS)
With reference to Figure 1, scarring in the bladder neck area of the vagina (between the red and blue vertical broken lines), takes away the elasticity needed for the opposite muscle forces (Figure 1, large red arrows), to function separately; the scar tissue “tethers” the stronger posterior pelvic forces, the LP/LMA to the anterior forces, PCM, and may overcome the PCM on effort. The balance of forces swings rapidly posteriorly; the posterior urethral wall is forcibly pulled open with consequent massive urine loss. There are two distinct causes of a scarred TVS, iatrogenic damage and obstetric fistula. Iatrogenic scarring sufficient to cause TVS, may occur after “native” vaginal repair, by large mesh sheets, and by Burch colposuspension. The typical symptom is massive urine loss which occurs immediately on getting out of bed in the morning. Continued day/night leakage after successful obstetric fistula repair, is attributed to the TVS (26), tethering of the opposite muscle forces by the massive scarring from the fistula. A skin graft applied to the bladder neck area of the vagina is curative for both the iatrogenic and obstetric fistula manifestations of TVS. Preventive use of the Singapore flap in Obstetric Fistula surgery has resulted in dramatically improved dryness, especially when performed in women who continue to lose massive amounts of urine after successful fistula closure (26).
Muscle or ligament, which is the main pathogenesis?
The pelvic floor functions in a holistic way. With reference to Figure 1, the ligaments, muscles, neurological circuits, somatic and visceral nerves work holistically to cortically control the bladder and the anorectum. Because damaged muscles cannot be surgically cured, the high cure rates reported for bladder/bowel/pain/prolapse dysfunctions by pelvic ligament repair indicate that pelvic ligaments are the most vulnerable of the different parts of the control system (13-18). This was directly demonstrated in an experiment with 47 blinded pelvic muscle biopsies in women with SUI (27), who were having a MUS operation. The sling cured 89% of the SUI the next day, despite evidence of prior severe muscle damage in the vast majority of the muscle biopsies. However, MUS ligament repairs never cure 100% of pelvic symptoms. It is, therefore, reasonable to attribute at least some of the failed symptom cures following ligament repairs to the muscle damage found, for example, in the blinded trial (28) and previous neurological studies by Swash et al. (28).
How to address striated muscle damage?
Patricia Skilling’s squatting-based pelvic floor exercises strengthen the three reflex pelvic muscles and the ligaments against which they contract. They improved individual bladder/bowel/pain symptoms by 50% in 60–90% of (mostly) premenopausal women (29). They can be used preoperatively, and postoperatively to strengthen the pelvic striated muscles, but work best in premenopausal women.
Role of smooth muscle in function and dysfunction
Though the emphasis in this work has been on the co-ordinated cortically-controlled interactions of ligaments and striated muscles, longitudinal smooth muscle contraction (in particular) of the vagina, the bladder and the urethra is stated as important for bladder control (30). We see organ smooth muscle working reflexly (and appropriately) in co-ordination with the binary control system during both closure and urination (31,32). For urination, the posterior vagina, and posterior urethral wall act like a trapdoor opened out prior to micturition by the LP/LMA (Figure 1). For continence during effort, the PCM (Figure 1) pulls the contracted distal vaginal wall forwards like a trapdoor to close the distal urethra from behind.
Conclusions
Anatomical defects anywhere in the binary system can alter peripheral, neurological control of the muscle forces by shifting the balance point 0-0 forwards or backwards to cause dysfunctions of opening or closure. The ligaments and the vagina are most vulnerable to childbirth and age because collagen is their main structural component. Clearly, however, damaged pelvic muscles must also play some role in bladder dysfunctions as it has been amply demonstrated, that they, too, can be damaged at childbirth.
Acknowledgments
We would like to express our gratitude to Editors Professor Peter Petros and Vani Bardetta for their exceptional support in the design and refinement of the article.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the International Society for Pelviperineology for the series “Integral Theory Paradigm” published in Annals of Translational Medicine. Peter Petros (Editor) and Vani Bardetta (Assistant Editor) served as the unpaid Guest Editors of the series. The article has undergone external peer review.
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-23-1771/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-23-1771/coif). The series “Integral Theory Paradigm” was commissioned by the International Society for Pelviperineology without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All clinical procedures described in this study were performed in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for the publication of this article and accompanying images. Human participation in the video was by patient permission on the basis it was deidentified.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
1A tap is turned on for the woman to hear. She washes her hands in a bowl during the cystometry.
References
- Barrington FJF. The component reflexes of micturition in the cat. Part III. Brain 1941;64:239-43. [Crossref]
- Feneley RCL, Harrison SCW. Urinary continence and micturition. In: Henry MM, Swash M. editors. Colpoproctology and the Pelvic Floor. 2nd ed. Oxford: Butterworths Heinermann; 1992:98-111.
- Fearnsides EG. The innervation of the bladder and urethra. Brain 1917;40:149-86. [Crossref]
- Parker MM, Rose DK. Inhibition of the bladder. Arch Surg 1937;34S:828-38. [Crossref]
- Lapides J, Ajemian EP, Stewart BH, et al. Physiopathology of stress incontinence. Surg Gynecol Obstet 1960;111:224-31. [PubMed]
- Hodgkinson CP, Ayers MA, Drukker BH. Dyssynergic detrusor dysfunction in the apparently normal female. Am J Obstet Gynecol 1963;87:717-30. [PubMed]
- Youssef AF. Sphincter incontinence in the female: a new approach to its classification, diagnosis and treatment. Acta Obstet Gynecol Scand 1957;36:439-59. [Crossref] [PubMed]
- Ingelman-Sundberg A. Urinary incontinence in women excluding fistulas. Acta Obstet Gynecol Scand 1952;31:266-91. [Crossref] [PubMed]
- Griffiths DJ. Cerebral control of bladder function. Curr Urol Rep 2004;5:348-52. [Crossref] [PubMed]
- Petros PE, Ulmsten UI. An integral theory of female urinary incontinence. Experimental and clinical considerations. Acta Obstet Gynecol Scand Suppl 1990;153:7-31. [Crossref] [PubMed]
- Ulmsten U, Petros P. Intravaginal slingplasty (IVS): an ambulatory surgical procedure for treatment of female urinary incontinence. Scand J Urol Nephrol 1995;29:75-82. [Crossref] [PubMed]
- Petros PE, Ulmsten UI, Papadimitriou J. The autogenic ligament procedure: a technique for planned formation of an artificial neo-ligament. Acta Obstet Gynecol Scand Suppl 1990;153:43-51. [Crossref] [PubMed]
- Nakamura R, Yao M, Maeda Y, et al. Retropubic tissue fixation system tensioned mini-sling carried out under local anesthesia cures stress urinary incontinence and intrinsic sphincter deficiency: 1-year data. Int J Urol 2017;24:532-7. [Crossref] [PubMed]
- Petros PE. New ambulatory surgical methods using an anatomical classification of urinary dysfunction improve stress, urge and abnormal emptying. Int Urogynecol J Pelvic Floor Dysfunct 1997;8:270-7. [Crossref] [PubMed]
- Liedl B, Inoue H, Sekiguchi Y, et al. Is overactive bladder in the female surgically curable by ligament repair? Cent European J Urol 2017;70:53-9. [PubMed]
- Goeschen K, Gold D, Liedl B, et al. Non-Hunner’s interstitial cystitis (IC) is different from Hunner’s IC and may be curable by uterosacral ligament repair. Urol Int 2022;106:649-57. [Crossref] [PubMed]
- Inoue H, Nakamura R, Sekiguchi Y, et al. Tissue Fixation System ligament repair cures major pelvic organ prolapse in ageing women with minimal complications - a 10-year Japanese experience in 960 women. Cent European J Urol 2021;74:552-62. [PubMed]
- Petros P, Abendstein B, Swash M. Uterosacral ligament repair improves urinary retention and other Fowler's syndrome descriptions. Cent European J Urol 2018;71:436-43. [PubMed]
- May RM. Simple mathematical models with very complicated dynamics. Nature 1976;261:459-67. [Crossref] [PubMed]
- Petros PE, Ulmsten U. Bladder instability in women: a premature activation of the micturition reflex. Neurourol Urodyn 1993;12:235-9. [Crossref] [PubMed]
- Papa Petros PE. Detrusor instability and low compliance may represent different levels of disturbance in peripheral feedback control of the micturition reflex. Neurourol Urodyn 1999;18:81-91. [Crossref] [PubMed]
- Lose G. Impact of changes in posture and bladder filling on the mechanical properties of the urethra in healthy and stress incontinent females. Neurourol Urodynam 1990;9:459-69. [Crossref]
- Constantinou CE, Govan DE. Contribution and timing of transmitted and generated pressure components in the female urethra. Prog Clin Biol Res 1981;78:113-20. [PubMed]
- Fowler CJ, Christmas TJ, Chapple CR, et al. Abnormal electromyographic activity of the urethral sphincter, voiding dysfunction, and polycystic ovaries: a new syndrome? BMJ 1988;297:1436-8. [Crossref] [PubMed]
- Tanagho EA. The anatomy and physiology of micturition. Clin Obstet Gynaecol 1978;5:3-26. [Crossref] [PubMed]
- Browning A, Williams G, Petros P. Skin flap vaginal augmentation helps prevent and cure post obstetric fistula repair urine leakage: a critical anatomical analysis. BJOG 2018;125:745-9. [Crossref] [PubMed]
- Petros PE, Swash M, Kakulas B. Stress urinary incontinence results from muscle weakness and ligamentous laxity in the pelvic floor. Pelviperineology 2008;27:107-109.
- Swash M, Snooks SJ, Henry MM. Unifying concept of pelvic floor disorders and incontinence. J R Soc Med 1985;78:906-11. [Crossref] [PubMed]
- Skilling PM, Petros P. Synergistic non-surgical management of pelvic floor dysfunction: second report. Int Urogynecol J Pelvic Floor Dysfunct 2004;15:106-10; discussion 110. [Crossref] [PubMed]
- Kato MK, Muro S, Kato T, et al. Spatial distribution of smooth muscle tissue in the female pelvic floor and surrounding the urethra and vagina. Anat Sci Int 2020;95:516-22. [Crossref] [PubMed]
- Petros PE, Ulmsten U. Role of the pelvic floor in bladder neck opening and closure I: muscle forces. Int Urogynecol J Pelvic Floor Dysfunct 1997;8:74-80. [Crossref] [PubMed]
- Papa Petros PE, Ulmsten U. Role of the pelvic floor in bladder neck opening and closure II: vagina. Int Urogynecol J Pelvic Floor Dysfunct 1997;8:69-73. [Crossref] [PubMed]


