
Underactive bladder, Fowler’s syndrome are potentially curable by uterosacral ligament repair
Highlight box
Key findings
• Underactive bladder (UAB) is potentially curable by uterosacral ligament (USL) repair.
What is known and what is new?
• UAB, Fowler’s syndrome (FS) are said to have no known cause or cure.
• UAB, FS, are likely part of the posterior fornix syndrome (PFS).
What is the implication, and what should change now?
• If UAB and FS are preoperatively demonstrated to be part of PFS, they are potentially curable by USL repair.
• If a tampon or probe in the posterior vaginal fornix improves emptying, minimally-invasive USL surgical repair could be considered.
Introduction
The key points of the article are summarized in the video abstract (Video S1).
Underactive bladder (UAB) in the female, is not well defined, and not well understood, especially as the prostatic urinary obstructed syndrome seems very different from that of the female. Another point of confusion is that UAB does not correlate very well with urodynamic “detrusor underactivity” (DU). UAB is said to be a common clinical entity, occurring in up to 45% of females depending on definitions used (1). Prevalence increases significantly in elderly women.
According to the International Continence Society (ICS), UAB syndrome is “characterized by a slow urinary stream, hesitancy, and straining to void, with or without a feeling of incomplete bladder emptying sometimes with storage symptoms” (2).
The etiology and pathophysiology underlying UAB is said to be unknown. Based on impaired bladder emptying characteristics, it has been hypothesized that UAB can arise from damage or malfunction of peripheral afferent, efferent, or central nervous system pathways, and detrusor myopathy (3).
A recent retrospective study of 1,726 patients said to have UAB, with the diagnosis based on patient history, estimated that 11.5% of patients would fall into the idiopathic subclass, with neurogenic causes accounting for 84.6% and myogenic causes 2.6% (4). Neurogenic causation is not the experience of the authors. As will be demonstrated later, high cure rates have been achieved with uterosacral ligament (USL) repair in a substantial number of women with bladder emptying difficulties.
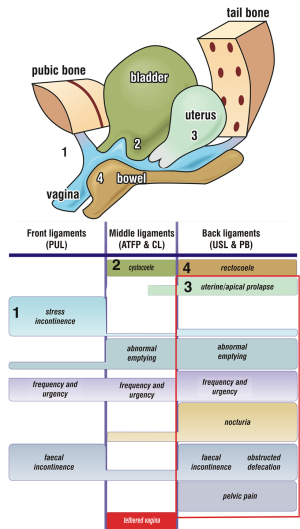
The aim of this review is to examine UAB from the perspective of the posterior fornix syndrome (PFS), of which UAB forms a part (5). PFS was first described in 1993 by Petros and Ulmsten (5). It consists of predictably co-occurring symptoms of urge, frequency, nocturia, chronic pelvic pain, abnormal emptying and raised post-void residual, caused by laxity in the USLs and cured or improved by surgical repair of the USLs (Figure 1, rectangle). It can also be improved non-surgically by strengthening pelvic muscle and ligaments with squatting-based exercises (7), and by tampons or devices which give mechanical support of the USLs.
UAB
From the perspective of PFS (5) and the Integral Theory Paradigm (ITP) (8), UAB is essentially an inability of the bladder to properly empty because of the inability of the posterior pelvic muscles to externally open the urethra prior to micturition. Symptoms of UAB are slow stream, intermittent stream (stopping and starting) hesitancy (difficulty starting), feeling of incomplete emptying, and post-micturition dribble. These symptoms form part of the PFS (5). The only well described clinical entity related to inability of the bladder to properly empty is Fowler’s syndrome (FS) (9). FS was originally described by Clare Fowler in young nulliparous women as urinary retention, characteristic electromyographic (EMG) abnormality in the striated urethral sphincter muscle and associated polycystic ovarian disease (PCOD). Later these EMG patterns were found in normal women and PCOD association was also discounted, which left only urinary retention. If we absent these now discounted original FS features, PCOD and specific EMG patterns, FS is essentially another form of UAB, which is, in turn, part of the PFS, as defined (5).
The anatomy of micturition and UAB
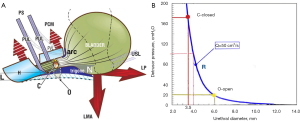
In the normal state, the opposite muscle forces (large arrows), close the urethral distally and also, at the bladder neck (Figure 2) (8). External opening of the urethra by pelvic muscle forces precedes normal micturition (8): the forward vector, pubococcygeus muscle (PCM) relaxes (signified by broken PCM arrows) and is accompanied by a fall in proximal urethral pressure (10); PCM relaxation releases the forward force applied by the vagina, “H”, to the posterior urethral wall; this allows the backward/downward the levator plate/conjoint longitudinal muscle of the anus (LP/LMA) muscle forces to pull open the posterior wall of the urethra, opening it from “C” (closed) to “O” (open), to facilitate micturition. The pubovesical ligament (PVL) contracts against the arc of Gilvernet on the anterior wall of the bladder, to prevent it prolapsing inwards against the combined backward/downward forces exerted by LP/LMA.
The flow mechanics of micturition and UAB
In Figure 2 the pressure/flow chart graphically demonstrates the effect of external opening of the urethra on the resistance to urine flow “R”. “R” is exponentially determined inversely to the fourth power of the radius (Poiseuille’s Law) (11-16). For example, if the urethra can be opened by the LP/LMA from “C” (closed) to “O” (open) even as little as 0.5 mm (say from 3.5 to 4 mm) (Figure 2), the pressure required for the detrusor to expel urine falls from 180 to 100 cm H2O for a 50 mL/s flow rate. If the urethra can be opened out to almost double this diameter, say from 3.5 to 6 mm, only 20 cm H2O detrusor pressure is required to empty the bladder at a 50 mL/s flow rate.
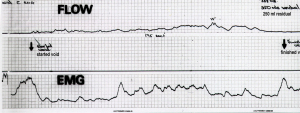
Figure 3 is from a video X-ray study of micturition in a normal patient. It compares the position of the organs at rest with normal micturition against vertical and horizontal co-ordinates taken from bony points. Note the backward and downward stretching of the rectum, vagina and bladder during micturition, which can only be explained by external muscle forces acting on the organs. Note also how the distal segment of the urethra appears to have been pulled backwards behind the vertical co-ordinate, consistent with relaxation of the forward vector and consequent backward stretching by the backward vectors. The posterior urethral wall has been significantly opened out, perhaps to double the original diameter. The EMG chart in Figure 4, taken from a cylindrical EMG probe in the posterior fornix of the vagina, confirms that the pelvic muscle contracts to open the urethra preceding the outflow of urine.
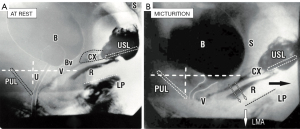
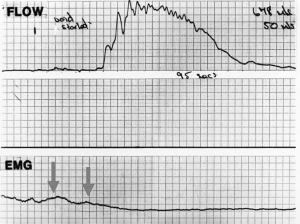
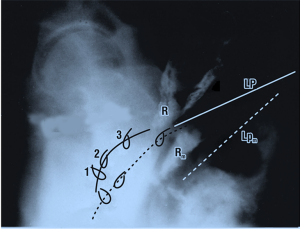
Figure 5 comprises superimposed rest/straining organ and myography with X-rays and vascular clips applied to the vagina at midurethra “1”, bladder neck “2” and bladder base “3”. During micturition, the clips, the vagina (broken lines) and the rectum Rm seem to be forcibly stretched backwards and downwards apparently by downward angulation of the LP moving to LPm (LP at micturition; broken lines). These organ movements mirror what is shown in Figure 2, two external muscle forces stretching the vagina, bladder base and posterior urethral wall backwards and downwards to open out the outflow tract. Opening out the urethra exponentially reduces urethral resistance to flow and so facilitates urine evacuation by detrusor contraction (11-16).
In a normal woman, once the urine starts flowing, the urine in the urethra being incompressible, resists the elastic closure forces of the urethra and surrounding tissues and removes the need for the pelvic muscles to continue to contract. In Figure 4, the disappearance of the EMG signal indicates that the pelvic muscles cease to contract as the detrusor contraction is now sufficient to expel the urine.
Pathogenesis of urinary retention and bladder emptying dysfunctions
With reference to Figure 2, if the USLs are weak, whether congenitally (for example in FS), injury at childbirth or by collagen breakdown after the menopause, the LP/LMA muscles which contract against them weaken, and the system becomes unbalanced. Compensatory forward-acting closure muscle action by the PCM narrows the distal urethra. The detrusor expulsion pressure needs to rise, and the patient experiences symptoms of “underactive bladder”. Perhaps a better description is “obstructed micturition”.
In Figure 6, the urodynamic flow chart and EMG are from a woman with urinary retention (or “UAB”). The flow is very slow and prolonged; according to underlying theory (8), this pattern indicates detrusor contraction against an unopened urethra.
Urinary retention: tiring of the external opening mechanism
Two important anatomical causes of abnormal bladder emptying, according to the underlying theory (8) and Figure 1 are cystocele and uterine prolapse. This is pictorially evident in the figure surmounting the diagnostic algorithm: lax USLs will weaken the opening forces LP/LMA; a cystocele will dissipate the muscle forces which stretch the anterior vaginal wall backwards to open the urethra.
External opening out of the outflow tract (Figure 2) exponentially lowers the urethral resistance to flow, inversely by the 4th power of the radius (Poiseuille’s Law) (11-13). It can be assumed, that the difference between the flow charts, Figure 4 (normal) and Figure 6 (obstructed), is failure of the external mechanism to adequately open out the urethra. Repeated traction by the striated muscles, as shown in the EMG (Figure 6), would lead to tiring of the opening muscles, resulting in the bladder not being fully emptied, and retention. Observation of the video resting and micturition X-rays (Figure 3) shows a doubling of the urethral diameter by what are clearly external muscle forces. As demonstrated in the pressure flow chart (Figure 2), the expulsion pressure required for a flow of 50 mL/s between a diameter of 3.5 and 6 mm falls from 180 to 20 cm H2O. In a mathematical model, Bush et al. calculated, that in order to achieve the funnel shape in Figure 3 without external opening, a detrusor pressure 2 orders of magnitude, 100 times greater than 160 cm H2O would be required, equivalent to a column 160 metres high (13).
Surgical and non-surgical treatment of non-iatrogenic urinary obstruction
Strengthening the USL, if possible, non-surgically (7), or surgically (15,17-20), can cure or improve UAB symptoms, presumably by restoring the contractile power of LP/LMA which contract against the USLs. The LP/LMA now more easily opens out the posterior urethral wall to reduce resistance to urine flow by bladder contraction. Using squatting-based pelvic floor exercises, Skilling reported that 23 women with pre-treatment mean residual urines of 202 mL (range, 50–550 mL), were reduced post-treatment to 71 mL (range, 15–450 mL) (P≤0.005) (7). Following surgical repair of the USL with a TFS minisling tape, Petros et al. (14,15) reported reduction of pre-treatment residual urine from mean 271 mL preoperatively to 53 mL post-operatively (P<0.005) (15). Mean emptying time for this group (n=29) decreased from 41 s (12–130 s) to 31 s (7–130 s) (P<0.005) (13). Petros and Goeschen et al. reported similar findings (17,18).
Challenging UAB and FS criteria by USL sling surgery
In urodynamically validated surgical studies, Goeschen et al. (18), Petros (15,17), and Himmler et al. (20) followed PFS protocols for diagnosis and surgery to repair USL laxity, using posterior slings. All reported high surgical cure rates for the PFS conditions (Figure 1, red rectangle).
Conclusions
The urodynamically validated surgical data indicate FS and UAB are most likely a part of the PFS, which are therefore, potentially curable by repair or mechanical support of USLs.
Acknowledgments
We would like to express our gratitude to Editors Professor Peter Petros and Vani Bardetta for their exceptional support in the design and refinement of the article.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the International Society for Pelviperineology for the series “Integral Theory Paradigm” published in Annals of Translational Medicine. Peter Petros (Editor) and Vani Bardetta (Assistant Editor) served as the unpaid Guest Editors of the series. The article has undergone external peer review.
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-23-1775/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-23-1775/coif). The series “Integral Theory Paradigm” was commissioned by the International Society for Pelviperineology without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All clinical procedures described in this study were performed in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for the publication of this article and accompanying images. Human participation in the video was by patient permission on the basis it was deidentified.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yamany T, Elia M, Lee JJ, et al. Female underactive bladder - Current status and management. Indian J Urol 2019;35:18-24. [Crossref] [PubMed]
- Chapple CR, Osman NI, Birder L, et al. Terminology report from the International Continence Society (ICS) Working Group on Underactive Bladder (UAB). Neurourol Urodyn 2018;37:2928-31. [Crossref] [PubMed]
- Cohn JA, Brown ET, Kaufman MR, et al. Underactive bladder in women: is there any evidence? Curr Opin Urol 2016;26:309-14. [Crossref] [PubMed]
- Li X, Liao LM, Chen GQ, et al. Clinical and urodynamic characteristics of underactive bladder: Data analysis of 1726 cases from a single center. Medicine (Baltimore) 2018;97:e9610. [Crossref] [PubMed]
- Petros PE, Ulmsten U. The posterior fornix syndrome: a multiple symptom complex of pelvic pain and abnormal urinary symptoms deriving from laxity in the posterior fornix. Scand J Urol Nephrol 1993;27:89-93.
- Petros P. The female pelvic floor function, dysfunction and management according to the Integral Theory. 3rd ed. Heidelberg: Springer Berlin; 2010.
- Skilling PM, Petros P. Synergistic non-surgical management of pelvic floor dysfunction: second report. Int Urogynecol J Pelvic Floor Dysfunct 2004;15:106-10; discussion 110. [Crossref] [PubMed]
- Petros PE, Ulmsten UI. An integral theory of female urinary incontinence. Experimental and clinical considerations. Acta Obstet Gynecol Scand Suppl 1990;153:7-31. [Crossref] [PubMed]
- Fowler CJ, Christmas TJ, Chapple CR, et al. Abnormal electromyographic activity of the urethral sphincter, voiding dysfunction, and polycystic ovaries: a new syndrome? BMJ 1988;297:1436-8. [Crossref] [PubMed]
- Tanagho EA, Meyers FH, Smith DR. Urethral resistance: its components and implications. II. Striated muscle component. Invest Urol 1969;7:195-205. [PubMed]
- Bush MB, Petros PE, Barrett-Lennard BR. On the flow through the human female urethra. J Biomech 1997;30:967-9. [Crossref] [PubMed]
- Papa Petros PE, Bush MB. A mathematical model for micturition gives new insights into pressure measurement and function. Int Urogynecol J Pelvic Floor Dysfunct 1998;9:103-7. [Crossref] [PubMed]
- Bush MB, Moron C, Messner-Pellenc L, et al. A mechanical model for the opening of the human female urethra. Proceedings of Biomedical Engineering 2005. Acta Press; 2005:210-3.
- Petros P, Lynch W, Bush M. Surgical repair of uterosacral/cardinal ligaments in the older female using the Tissue Fixation System improves symptoms of obstructed micturition and residual urine. Pelviperineology 2015;34:112-6.
- Petros P, Abendstein B, Swash M. Retention of urine in women is alleviated by uterosacral ligament repair: implications for Fowler's syndrome. Cent European J Urol 2018;71:436-43. [PubMed]
- Bush MB, Liedl B, Wagenlehner F, et al. A finite element model validates an external mechanism for opening the urethral tube prior to micturition in the female. World J Urol 2015;33:1151-7. [Crossref] [PubMed]
- Petros PE. New ambulatory surgical methods using an anatomical classification of urinary dysfunction improve stress, urge and abnormal emptying. Int Urogynecol J Pelvic Floor Dysfunct 1997;8:270-7. [Crossref] [PubMed]
- Goeschen K, Gold DM. Surgical cure of chronic pelvic pain, associated bladder & bowel symptoms by posterior sling in 198 patients validates the Pescatori Iceberg principle of pelvic symptom co-occurrence. Pelviperineology 2017;36:84-8.
- Petros PEP, Richardson PA. TFS posterior sling improves overactive bladder, pelvic pain and abnormal emptying, even with minor prolapse. A prospective urodynamic study. Pelviperineology 2010;29:52-5.
- Himmler M, Kohl M, Rakhimbayeva A, et al. Symptoms of voiding dysfunction and other coexisting pelvic floor dysfunctions: the impact of transvaginal, mesh-augmented sacrospinous ligament fixation. Int Urogynecol J 2021;32:2777-86. [Crossref] [PubMed]


