
Structural, functional, and dysfunctional pelvic anatomy
Highlight box
Key findings
• Control of the bladder and rectum is from outside the organ, from muscles and ligaments, with ligaments the most vulnerable to damage because of collagen loss by birthing and age.
What is known and what is new?
• Prevalence of vaginal excision for prolapse indicates belief that the vagina is the cause; the role of ligaments is ignored.
• The role of collagen in pelvic floor function and dysfunction is fundamental.
• Ligaments support organs with collagen, their main structural component.
• Collagen is vulnerable to damage from childbirth and deterioration by age.
• Defective ligament collagen causes both symptoms and prolapse.
What is the implication and what should change now?
• There is a distinct difference in collagen ligament content between premenopausal and postmenopausal women.
• Native ligament repairs work satisfactorily in premenopausal women.
• For older women, collagen creation methods such as slings provide a higher cure rate and should be considered.
Introduction
The 4-minute video abstract (Video S1) summarizes the key anatomical points; viewing it a priori is recommended.
The aim of this contribution to the ATM Integral Theory Paradigm (ITP) issue is to describe the structural anatomy of the pelvic muscles and ligaments and the pathways leading to ligament damage sufficiently to lay down an anatomical foundation for the ITP papers which follow.
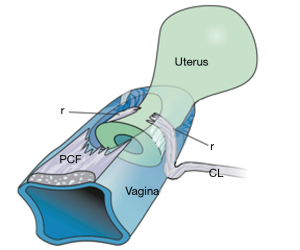
With reference to Figure 1 (1), the structural basis of the Integral Theory (2) was the discovery that:
- Four main pelvic muscles, pubococcygeus, levator plate, conjoint longitudinal muscle of the anus, puborectalis and five main pelvic ligaments, pubourethral, cardinal, uterosacral, arcus tendinous fascia pelvis, and perineal body interact to maintain pelvic organ structure and function (Figure 1).
- Ligaments structurally support organs and are the most vulnerable to damage.
- The vagina is structurally weak; its main role is for function, and should be conserved, not excised.
- Weakening of collagen, the main structural component of ligaments, is the main cause of organ prolapse and lower urinary tract symptoms (LUTS).
- Strengthening damaged ligaments, whether surgically or non-surgically, can improve or cure symptoms.
- For postmenopausal women, collagen-creating techniques to strengthen ligaments are advised.
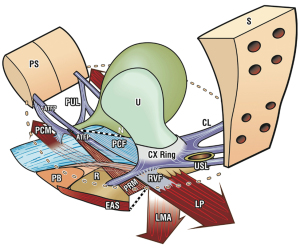
With reference to Figure 1, the functional basis of the Integral Theory (2) was the discovery that:
- Three oppositely acting reflex muscles, pubococcygeus (PCM), levator plate (LP), and conjoint longitudinal muscle of the anus (LMA) contract against the pubourethral (PUL) and uterosacral ligaments (USL) to control urethral closure, evacuation and to support bladder stretch receptors “N” from activating premature emptying (“urge incontinence”) (Figure 1). Video S2 (ultrasound) shows the action of three reflex directional muscle forces, forwards, backwards, downwards. A fourth muscle, puborectalis, reflexly joins the other three to help enact anorectal closure and evacuation (3) (Video S3).
The muscles
There are four main pelvic muscles (Figures 1,2). Three pelvic muscles, PCM, LP and conjoint LMA are entirely reflex (Figure 1, large arrows). The PCM attaches to the lateral part of the distal vagina and to the descending part of the PUL (4). Its medial part contracts forwards against the PUL (Figures 1,2). Its lateral part contracts backwards against the PUL, sweeps backwards to join its contralateral muscle to join with iliococcygeus to form the LP (Figure 2). LMA takes fibres from the LP, PCM, and inserts into the external anal sphincter (EAS) (Figure 2). The LMA contracts downwards against the USL (Figure 1). The puborectalis muscle (PRM) contracts directly against the pubic symphysis (PS) (Figures 1,2). It acts as both a voluntary muscle (squeezing upwards “Kegel”) and as a reflex muscle to contract for anorectal closure and relax for defecation.
The ligaments
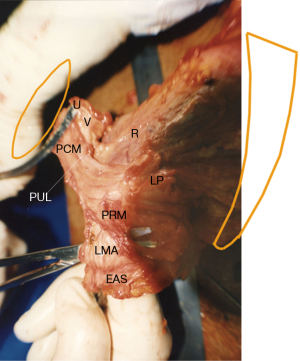
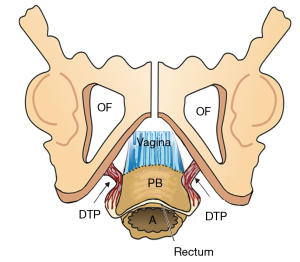
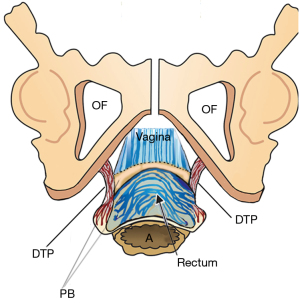
The ligaments (Figure 1) contain nerves, blood vessels and smooth muscle (2) and so they actively participate in all the functions of the pelvic muscles which pull against them. Collagen is the main structural component of ligaments (2). With reference to Figure 1, the PUL is a short ligament, about 4 cm long in vivo, and attaches to the mid urethra, also to the PCM and the distal vagina. USLs originate from S2–4, are attached loosely to the lateral walls of the rectum by fine ligamentous structures and insert into the posterior wall of the cervix. At a distance, 2 cm from the cervix, the USLs support the pelvic visceral plexus ganglions, sympathetic (T11–L2) and parasympathetic (S2–4). The arcus tendineus fascia pelvis (ATFP) suspends the vagina laterally, arising just above the PUL behind the symphysis and inserting into the ischial spine. In vivo, the USLs are about 9–10 cm long. With reference to Figure 3, the perineal body is suspended by the deep transversus perinei ligaments. These are about 4 cm long and insert behind the junction of the upper 2/3 and lower 1/3 of the descending ramus (5,6).
Simplistically, ligaments are the structural components of the pelvic floor, while the vagina is concerned with the function of transmitting the muscle forces which open and close the urethra (2). The collagen in ligaments is collagen 1, which is much stronger than the collagen 3, and elastin which give the vagina its elasticity. The differential strengths of structure, ligaments with a breaking strain 300 mg/mm2 and the vagina with 60 mg/mm2, reflect the different roles of each (7).
The vagina
The vagina is the emptying tube for the uterus, fetus and menstrual blood. A tensioned vagina controls the afferent bladder evacuation signals from urothelial stretch receptors “N” being stretched by the PCM and the LP to support “N” from below (Figure 1). “N” are sensitive to the hydrostatic pressure of the urine. The vaginal tension below “N” is regulated by muscle spindles in the PCM and LP. The tension must be sufficient to stop the nerves from firing off afferent emptying impulses, but sufficiently elastic to allow separate closure of the distal urethra by PCM and the bladder neck by the LP/LMA.
The urethra and anus
Urethra and anus are composed of smooth muscle, collagen, and elastin. They are the emptying tubes for bladder and rectum. They are closed for continence and opened for evacuation by the external, cortically directed action of the four pelvic muscles (Figure 1). Anal stretch receptors are hypothesized to act similarly to those of the urothelium and are considered to be situated at a level just above the insertion of the LP into the posterior wall of the rectum (Figure 1).
Resources for further reading
See Petros and Ulmsten’s studies (https://obgyn.onlinelibrary.wiley.com/toc/16000412/1990/69/S153) for bladder function and dysfunction (2).
See Petros and Swash’s study (https://www.researchgate.net/publication/267778578_The_MusculoElastic_Theory_of_anorectal_function_and_dysfunction) for anorectal function and dysfunction (3).
Role of muscles/ligaments in continence control
The bladder and bowel have only two modes, open or closed; these are cortically directed. The pelvic muscles externally close the bladder/bowel emptying tubes for continence, externally open them for evacuation (Figure 1, broken lines). The directional muscles stretch the organs bilaterally to prevent the bladder/bowel stretch receptors from prematurely activating micturition and defecation (“urge incontinence”) (2,3).
Urethral closure on effort
With reference to Figure 1, forward contraction of the PCM stiffens the posterior wall of the distal vagina, distal urethra and the PUL; the LP stretches the proximal vagina and urethra backwards to stiffen them; the LMA pulls the now semi-rigid bladder base down against USLs to rotate it downwards to close the urethra at the bladder neck (like kinking a garden hose) (see forward closure of urethra, Video S2).
Anorectal closure on effort
With reference to Figure 1, forward contraction of the PRM stiffens the anterior wall of the rectum; forward contraction of the PRM stiffens the posterior wall of the rectum; the LP contracts backwards against the PUL to stretch the rectum backwards to render it semi-rigid; the LMA pulls against USLs to rotate the now semirigid rectum downwards around the contracted PRM to close the anorectal angle, kinking the rectum like a garden hose (see anorectal closure action of pelvic muscles, Video S3).
Micturition
Once the micturition reflex is activated, the patient feels an urge to go. The PCM relaxes, taking the forward pressure off the distal vagina and this allows the LP/LMA to stretch the vagina, trigone and bladder base backwards and downwards to open out the posterior wall of the urethra, exponentially lowering the resistance to urine flow, to enable emptying (2,8) (see micturition xray myogram, Video S4).
Defecation
The process for defecation is very similar to the micturition process, except that it is the PRM which relaxes. With reference to Figure 1, when the PRM relaxes, the LP/LMA pull open the posterior wall of the anorectum (broken lines). The PRM continues contracting to stiffen the anterior wall of the anorectum. External opening of the neorectum by pelvic muscles immediately prior to defecation lowers the intra-anal resistance to enable emptying (3,9) (see Video S5).
Anatomical pathogenesis of structural dysfunctions
According to the ITP, the key concept in pathogenesis of structural dysfunctions of the pelvic floor is ligament weakness caused by collagen deficiency. The main causes of collagen weakening are pregnancy and childbirth and collagen degeneration after the menopause. In a small group of women, congenital looseness of ligaments may also cause dysfunction.
Ligament overdistension may affect prolapse, symptoms, muscle/bone attachments
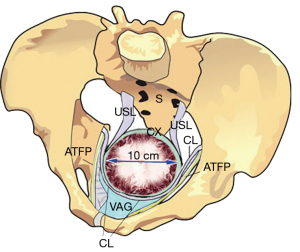
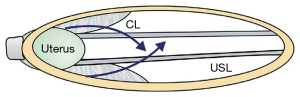
Though the ligament collagen has been softened by relaxin and other hormones, a head at full dilatation (10 cm) places enormous pressure on the proximal 5 cm of the ligaments surrounding it. With reference to Figure 4, the cardinal ligaments (CLs), USL, and the attachments of the pubocervical fascia layer of the vagina may stretch or rupture at childbirth. The CL stretching or rupture may cause transverse defect cystocele (Figure 5). USLs may elongate to cause uterine prolapse (Figure 6).
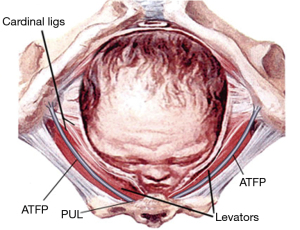
With reference to Figure 7, the expanding head even when fully flexed (9.4 cm) places enormous pressure on the PUL and the pelvic muscles at their insertion to the symphysis to cause stress urinary incontinence (SUI) and dislocated PCM/PRM insertions to the symphysis (10) (Figure 8).
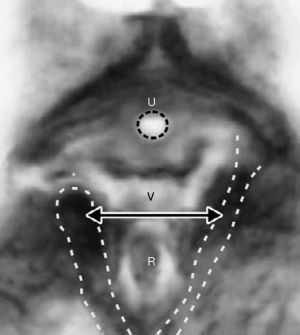
With reference to Figure 9, the perineal body, and its suspensory ligaments (deep transversus perinei), are especially vulnerable to over-stretching or even rupture. With a deflexed head (11.2 cm diameter), the pressure of the emerging head is far greater, and damage may occur to the vagina, rectum, perineal body muscle and ligamentous attachments.
With reference to Figure 10, at “3” the head (circle) can overstretch uterosacral (USL) ligaments to cause uterine prolapse and enterocele. If the lateral ligamentous attachments of the USL to the rectum “R” pull the anterior rectal wall forwards as USLs lengthen, the anterior rectal wall may be stretched forwards to cause rectal intussusception (11) (Figure 11), which can be cured by USL repair (12); at circle “2”, the CL elongation or rupture may cause cystocele’(transverse defect); at circle “4”, perineal damage may cause rectocele and descending perineal syndrome; at circle “1”, excess pressure on the levator muscles may dislocate or tear their collagenous insertions to symphysis pubis, while excess pressure on PUL may cause stress incontinence.
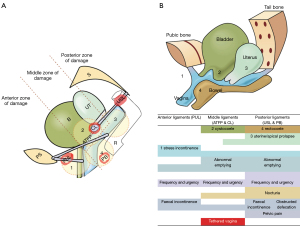
The damaged ligaments
The damaged ligaments fall into three natural zones (Figure 10). Anterior: external meatus to bladder neck; middle: bladder neck to cervix; posterior: cervix to introitus.
Damaged vaginal tissue
The vaginal “fascia” is the musculo-elastic layer of the vaginal wall. The anterior vaginal support of the urethra distally is called the “suburethral vaginal hammock”; more proximally, the vaginal support of bladder base is the “pubocervical fascia”. The posterior vaginal wall (rectovaginal or Denonvillier’s fascia) is attached proximally to the cervix and USLs, and is supported distally by the perineal body, which attaches it to the rectum.
The numbers in Figure 10A, causatively correlate with the same numbers in the diagnostic algorithm (Figure 10B), as regards prolapse, ligament damage and LUTS symptoms. For example, circle 3, damage to the USL may cause USL damage, uterine prolapse, and all the symptoms in the left zone.
Effects of age on native ligament repair
The dramatic effect of age and hormone withdrawal at menopause was demonstrated in a comparative study by Shkarupa et al. (13). Results at 18 months from native tissue plication of cardinal and USL gave 79.6% prolapse cure for premenopausal women but only 15.4% cure for postmenopausal women. The cure rates for urge and nocturia symptoms, were 87.7% and 67.3% against 17.9% and 15.4% respectively. The authors concluded that postmenopausal women required collagenopoietic slings for adequate repair of their prolapse and symptoms. This comment has been validated by many studies in older women using slings, some with large numbers (14-24), including high 5 years (14) and 10 years (15) cure rates for both pelvic organ prolapse (POP) and pelvic symptoms.
The anatomical basis of the ITP diagnostic system
The algorithm (Figure 10B), uses symptoms to diagnose damaged ligaments and infer the presence of prolapse, which is often minimal. Symptoms are ticked in each box where they occur, even if they occur only “sometimes”. The conditions in all three columns may be caused by laxity of ligament(s) in that zone.
Diagnosis of ligament damage is by deduction
With reference to the Diagnostic Algorithm (Figure 10B), nocturia, chronic pelvic pain, obstructive defecation are uniquely caused by posterior zone ligament defects (USL). Stress urinary or stress fecal incontinence are uniquely caused by anterior zone (PUL) defects. These zones are ticked first and are the starting point for diagnosis of ligament damage and inferring of specific prolapses. The algorithm is based on individual symptoms occurring in an individual patient. Definitions such as, “mixed incontinence”, “overactive bladder” (OAB), “Posterior Fornix Syndrome” (PFS), which combine often co-occurring symptoms have no place in this diagnostic algorithm. The individual symptoms from an individual patient with these conditions must be entered into the algorithm, so a diagnosis of ligament causation can be inferred. With reference to Figure 10B, SUI, when it co-occurs with urge is called “mixed incontinence”. The SUI part is caused by a loose/weak PUL, but the urge may be caused by ligament defects in any of the three zones. OAB (urge, frequency) may be caused by any zone, but if there is also nocturia, the main ligament damage is likely the USL in the posterior zone. As regards PFS symptoms (co-occurrence of urge, frequency, nocturia, abnormal emptying, and chronic pelvic pain), the presence of nocturia and abnormal emptying place causation firmly with the USL (note: often the worst PFS cases have minimal prolapse).
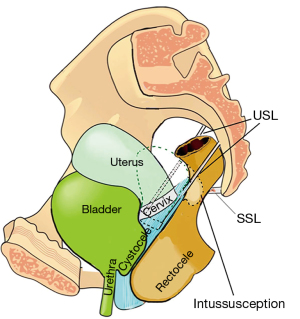
How uterine prolapse may cause anterior rectal intussusception
Examination of Figure 11 indicates how the attachment of USLs to the lateral wall of the rectum can cause intussusception in women who have uterine prolapse. As USLs lengthen, they splay laterally, causing the anterior rectal wall to also elongate laterally; such stretching weakens the rectal wall structurally. Its collagen concentration lessens and the anterior rectal wall invaginates to cause intussusception (11).
Conclusions
The structural basis of the Integral Theory is holistic. Four main pelvic muscles interact holistically with five main pelvic ligaments to maintain pelvic organ structure and function. As the vagina is a structurally weak organ, the support it provides to the bladder base is contingent on being stretched by opposite pelvic muscle forces with its main role being to transmit muscle forces to facilitate continence, evacuation and control of urgency. The vagina should not be excised as it cannot regenerate and, therefore, it should be conserved. The ligaments provide the main structural support for the organs and are the most vulnerable part of the anatomical system to injury because their structural collagen can be weakened during labour and collagen loss after the menopause due to collagen breakdown. Consequently, collagen loss is the main cause of organ prolapse and LUTS. By strengthening damaged ligaments, whether surgically or non-surgically, symptoms and prolapse can be improved or cured. The principal cause of dysfunction in older women is collagen loss in the ligaments; therefore, collagen-creating techniques are advised. Neoligaments can be created with precisely inserted tapes. Alternatively, ligaments can be strengthened by plicating them with wide-bore No. 2 or No. 3 polyester sutures.
Acknowledgments
We would like to express our thanks to the Editors, Professor Peter Petros and Vani Bardetta for their tremendous help in designing and modifying the article.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the International Society for Pelviperineology for the series “Integral Theory Paradigm” published in Annals of Translational Medicine. Peter Petros (Editor) and Vani Bardetta (Assistant Editor) served as the unpaid Guest Editors of the series. The article has undergone external peer review.
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-23-1877/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-23-1877/coif). The series “Integral Theory Paradigm” was commissioned by the International Society for Pelviperineology without any funding or sponsorship. M.N. reports stocks and stock options at Momentis and Femselect. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All clinical procedures described in this study were performed in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for the publication of this article, accompanying images and videos. Human participation in the videos was by patient permission on the basis it was deidentified.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Petros P. The female pelvic floor function, dysfunction and management according to the Integral Theory. 3rd ed. Heidelberg: Springer Berlin; 2010.
- Petros PE, Ulmsten U. An Integral Theory of female urinary incontinence. Acta Obstet Gynecol Scand 1990;69:1-79. [Crossref] [PubMed]
- Petros PE, Swash M. The Musculo-Elastic Theory of anorectal function and dysfunction. Pelviperineology 2008;27:89-121.
- Petros PE. The pubourethral ligaments--an anatomical and histological study in the live patient. Int Urogynecol J Pelvic Floor Dysfunct 1998;9:154-7. [Crossref] [PubMed]
- Wagenlehner FM, Del Amo E, Santoro GA, et al. Live anatomy of the perineal body in patients with third-degree rectocele. Colorectal Dis 2013;15:1416-22. [Crossref] [PubMed]
- Wagenlehner FM, Del Amo E, Santoro GA, et al. Perineal body repair in patients with third degree rectocele: a critical analysis of the tissue fixation system. Colorectal Dis 2013;15:e760-5. [Crossref] [PubMed]
- Yamada H. Aging rate for the strength of human organs and tissues. In: Evans FG, editor. Strength of biological materials. Baltimore: Williams & Wilkins Co; 1970:272-80.
- Bush MB, Moron C, Messner-Pellenc L, et al. A mechanical model for the opening of the human female urethra. In: Adlassnig KP, Bracale M, editors. Proceedings of Biomedical Engineering 2005, Austria. Acta Press; 2005:210-13.
- Petros P, Swash M, Bush M, et al. Defecation 1: Testing a hypothesis for pelvic striated muscle action to open the anorectum. Tech Coloproctol 2012;16:437-43. [Crossref] [PubMed]
- DeLancey JO, Kearney R, Chou Q, et al. The appearance of levator ani muscle abnormalities in magnetic resonance images after vaginal delivery. Obstet Gynecol 2003;101:46-53. [Crossref] [PubMed]
- Petros PEP. The biomechanics of uterine prolapse impact rectal intussusception, ODS and surgical restoration. Tech Coloproctol 2022;26:161-2. [Crossref] [PubMed]
- Abendstein B, Brugger BA, Furtschegger A, et al. Role of the uterosacral ligaments in the causation of rectal intussusception, abnormal bowel emptying, and fecal incontinence: A prospective study. Pelviperineology 2008;27:118-21.
- Shkarupa D, Zaytseva A, Kubin N, et al. Native tissue repair of cardinal/uterosacral ligaments cures overactive bladder and prolapse, but only in pre-menopausal women. Cent European J Urol 2021;74:372-8. [Crossref] [PubMed]
- Inoue H, Kohata Y, Fukuda T, et al. Repair of damaged ligaments with tissue fixation system minisling is sufficient to cure major prolapse in all three compartments: 5-year data. J Obstet Gynaecol Res 2017;43:1570-7. [Crossref] [PubMed]
- Inoue H, Nakamura R, Sekiguchi Y, et al. Tissue Fixation System ligament repair cures major pelvic organ prolapse in ageing women with minimal complications - a 10-year Japanese experience in 960 women. Cent European J Urol 2021;74:552-62. [Crossref] [PubMed]
- Piñango-Luna S, Level-Córdova L, Petros PE, et al. A low-cost artisan tension-free tape technique cures pelvic organ prolapse and stress urinary incontinence: Proof of concept. Cent European J Urol 2020;73:490-7. [Crossref] [PubMed]
- Piñango-Luna S, Level-Córdova L, Petros PE, et al. A low cost artisan tension-free tape technique cures pelvic organ prolapse and stress urinary incontinence - proof of concept. Cent European J Urol 2020;73:490-7. [Crossref] [PubMed]
- Wagenlehner F, Muller-Funogea IA, Perletti G, et al. Vaginal apical prolapse repair using two different sling techniques improves chronic pelvic pain, urgency and nocturia: a multicentre study of 1420 patients. Pelviperineology 2016;35:99-104.
- Caliskan A, Ozeren M, Goeschen K. Modified posterior intravaginal slingplasty: does the additional bilateral tape attachment to the sacrospinous ligament improve the results? Cent European J Urol 2018;71:326-33. [Crossref] [PubMed]
- Enache T, Bratila E, Abendstein B. Chronic pelvic pain of unknown origin may be caused by loose uterosacral ligaments failing to support pelvic nerve plexuses - a critical review. Cent European J Urol 2020;73:506-13. [Crossref] [PubMed]
- Petros P, Abendstein B. Pathways to causation and surgical cure of chronic pelvic pain of unknown origin, bladder and bowel dysfunction - an anatomical analysis. Cent European J Urol 2018;71:448-52. [Crossref] [PubMed]
- Petros P. A gynecological perspective of interstitial cystitis/bladder pain syndrome may offer cure in selected cases. Cent European J Urol 2022;75:395-8. [Crossref] [PubMed]
- Liedl B, Goeschen K, Grigoryan N, et al. The association between pelvic organ prolapse, pelvic pain and pelvic reconstructive surgery using transvaginal mesh: a secondary analysis of a prospective multicenter observational cohort trial. J Clin Gynecol Obstet 2020;9:79-95.
- Petros P, Richardson P. TFS posterior sling improves overactive bladder, pelvic pain and abnormal emptying, even with minor prolapse. A prospective urodynamic study. Pelviperineology 2010;29:52-5.


