
A brief physiology and pathophysiology of the bladder
Highlight box
Key findings
• Control of the bladder is not from the bladder itself, but from ligaments and muscles outside of it, with ligaments most vulnerable to damage because of collagen changes at birth and after menopause.
What is known and what is new?
• Overactive bladder (OAB) and emptying problems are said to have unknown pathogenesis and no cure.
• Bladder control is binary, either closed or open, cortically controlled, peripherally activated, by pelvic muscles contracting against suspensory ligaments to close urethra (continence), open it, and to stretch the vagina to prevent premature micturition (urgency).
• Pathogenesis of female urinary incontinence is mainly from outside the bladder, from weak or loose ligaments and in special circumstances, from nerve or muscle damage.
• OAB is a prematurely activated but otherwise normal micturition.
• Weak ligaments are an important cause of SUI, urgency (OAB), and emptying dysfunctions [underactive bladder (UAB)].
What is the implication, and what should change now?
• OAB, UAB, and SUI are potentially surgically curable by ligament repair. “Repair the structure (ligament) and you will restore the function”.
Introduction
The key points of the article are summarized in the video abstract (Video S1).
Foreword by Professor Axel Ingelman-Sundberg, Karolinska Institutet 1990:
“To me it has always been obvious, that, in general, the reason behind female urinary incontinence has to be looked for outside the bladder, that is, in the structures supporting the urethra and bladder neck—specifically ligaments, pelvic floor muscles and vagina. If symptoms of urinary incontinence arise from a dysfunctional anatomy in the aforementioned structures, then function should come with restoration of anatomy.”
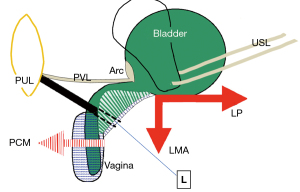
The remit of this review is confined to experimental works and publications relevant to the integral theory of female urinary incontinence (IT). Since its first publication in 1990, the IT has challenged the general view that the pathogenesis of overactive bladder (OAB) (urge, frequency, nocturia) is unknown and there is no cure. The key discovery in the 1990 integral theory was that the control of bladder function was not from the bladder itself, but from structures outside it, by three oppositely acting reflex striated pelvic muscle forces (1) (Figure 1A, large arrows) (see Video S2). These muscles contracted against suspensory ligaments, anterior pubourethral ligament (PUL) and posterior uterosacral ligament (USL), to close the urethra for continence, open it for evacuation, and to stretch the vagina like a trampoline to prevent excess impulses from the urothelial stretch receptors which may cause unwanted urgency (OAB). The remit of this review is based on integral theory publications including (but not confined to) references (1-18) (https://obgyn.onlinelibrary.wiley.com/toc/16000412/1990/69/S153).
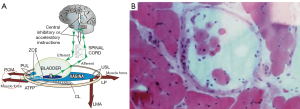
The binary model for normal bladder function
With reference to Figure1A, the binary control works like an electric switch to control two different reflexes, either closed or open (micturition) (2) (see Videos S2,S3). These reflexes are mutually exclusive, with cortical and peripheral components for each function. Muscle spindles work via a reflexly coordinated feedback system to maintain the balance of forces which tension the vagina (Figure 1B). Closure is the dominant reflex.
Control of urgency
The opposite contractions of the pubococcygeus muscle (PCM), and the levator plate (LP) and conjoint longitudinal muscle of the anus (LMA) tension the vagina to support the urothelial stretch receptors “N” from below (Figure 1A). Tensioning stretches the vaginal collagen in the manner of a trampoline to support the hydrostatic pressure exerted by the urine column on “N” (see Video S4). This reflex action prevents “N” firing off the afferent emptying impulses which activate the micturition reflex to empty, felt as “an urge to go” by the patient (1).
Urethral closure on effort
With reference to Figure 1A, the PCM pulls the distal vagina forwards against the PUL to close the urethra from behind; the LP stretches the proximal vagina and urethra backwards against the PUL to tension them; the LP contracts down against the USL to rotate the now tensioned bladder base around the arc of Gilvernet to close the urethra at the bladder neck (1) (see Video S2).
Micturition
With reference to Figure 1A (1) the PCM relaxes; the LP/LMA pull open the posterior wall of the urethra (white broken lines) to reduce resistance to urine flow from the contracting detrusor. Active opening by external muscle forces exponentially lowers the resistance to urine flow to enable evacuation (inversely by the 4th power of the radius “Poiseuille’s Law”) (see Video S3).
Pelvic muscle or ligaments, which is the main cause of bladder dysfunction?
The integral theory paradigm (ITP)’s main focus is loose or weak ligaments caused by altered collagen (1). Pathogenesis can be congenital, pregnancy/childbirth related (Figure 2) or menopausal (collagen breakdown/excretion). Clearly muscle damage must be a factor in pathogenesis. However, the high cure rates for pelvic symptoms attained following ligament repair (3) confirm the integral theory’s view that ligament damage is the main cause. A further validation of this view was a blinded biopsy study of 47 women undergoing a midurethral sling operation for stress incontinence; 44 women had gross histological muscle damage, yet 89% were cured of SUI the next day (4).
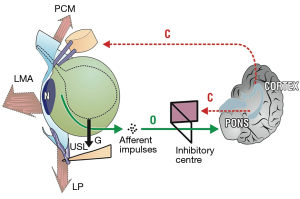
Pathogenesis within the binary model
With reference to Figure 1A, any abnormality in the binary control circuit can affect the micturition reflex to cause retention or OAB. Neurological lesions such as stroke (cortex), spinal cord injury, multiple sclerosis (MS) in the afferent circuit, can cause retention. MS in the efferent nerve circuits can affect peripheral control to cause urge. Local lesions such as inflammation or a tumor near “N” can increase afferent impulses to cause OAB and incontinence.
Urge as a prematurely activated uncontrolled normal micturition
A urodynamically controlled experiment demonstrated what was then known as “detrusor instability” (now “DO”) was equivalent to a prematurely activated micturition (5). The pattern of urine loss was identical to that seen in normal micturition: (I) sensation of urge; (II) fall in proximal urethral pressure; (III) rise in detrusor pressure; (IV) urine loss.
OAB (urge, frequency, nocturia)
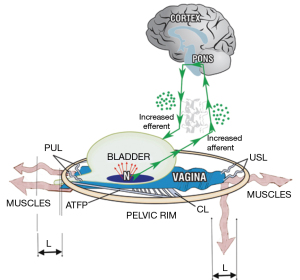
With reference to Figure 3, weakness in either the PUL or USL will weaken the muscles which contract against them. Weakened PCM and LP/LMA muscles cannot adequately tension the vagina to support “N” to prevent the afferent emptying impulses reaching the cortex which are interpreted as “urge to go”. Beyond a critical point the micturition reflex temporarily takes control, the system becomes unstable, and swings between “open” and “closed”, which is the key characteristic of OAB and urodynamic “DO” (“detrusor overactivity”) (6). See also https://doi.org/10.1002/nau.24990 (2).
Emptying: urinary retention
With reference to Figure 3, weak USLs weaken the contractile force of the LP and LMA muscles which contract against them. The weakened LP/LMA cannot open the urethra prior to micturition. Consequently, the detrusor contracts against a relatively unopened urethra against high urethral resistance, which the cortex interprets as “obstruction”, which is what it is (7,8).
The pathogenesis of nocturia
A loose USL cannot prevent the proximal vagina and bladder base from being pulled down by gravity “G” (Figure 4). The downward stretching pulls on the stretch receptors “N” which activate the micturition reflex which the cortex interprets as “urge”. A tampon can sometimes alleviate nocturia, if it can sufficiently support the weakened USLs.
Surgical cure of lower urinary tract symptoms (LUTS)
Following the first report of cure of SUI and urge by the prototype midurethral sling operation in the 1990 integral theory (1), a principal focus of the ITP has been surgical cure of prolapse and LUTS by ligament repair and to explain how restoring collagen to a damaged ligament could cure such a wide range of LUTS.
How surgery cures nocturia and urgency
With reference to Figure 4, reinforcing the USLs with a sling prevents bladder base traction by gravity “G” and so prevents the stretch receptors “N” firing off to cause urge. However, the peripheral musculoelastic control mechanism cannot counter bladder instability caused by damage to the cortical inhibitory circuits, say by MS, or excessive stimulation of “N” by tumor or inflammation. These conditions need to be excluded before proceeding to surgical correction.
The anatomy of continence and stress urinary incontinence (SUI)
The mechanics of continence and SUI are described in Figure 5. Direct proof of the importance of a firm midurethral anchoring point is control of SUI with hemostat support at the midurethra, evident in Video S5.
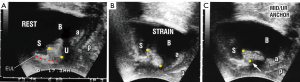
Ultrasound proofs of weak PUL as cause of SUI
Figure 6 is a transperineal ultrasound of a woman with SUI. At rest (Figure 6A) the urethra is closed. In Figure 6B (strain) the PUL extends. The anterior and posterior vaginal walls (a&p) are stretched backwards/downwards to open out the posterior wall of the urethra along its length. In Figure 6C (midurethral anchor) a hemostat (white arrow) inserted behind the symphysis as in Video S2, mechanically supports the weakened PUL, and temporarily restores urethral closure along its length and normal urethrovesical geometry on straining. The anterior and posterior vaginal walls (a&p) are tensioned; the urethra is closed at the bladder neck and distally.
Explaining surgical and non-surgical cure/improvement of OAB
“The reason behind female urinary incontinence has to be looked for outside the bladder, that is, in the structures supporting the urethra and bladder neck, specifically, ligaments, pelvic floor muscles and vagina” (1). Ligaments are the structures most vulnerable to deterioration because of changes in their main structural component, collagen, caused by labour, delivery and age (1). Since the early 1990s, surgeons who follow the ITP have been reporting high cure rates for SUI, OAB, urinary retention and chronic pelvic pain, by repair of the suspensory ligaments of the pelvis, principally the PUL and USL (9-18).
Conclusions
Bladder control is binary, with cortical and peripheral components. A small change in definition from “overactive” to “overactivated” is consistent with this concept, retains the acronym “OAB”, and opens the door to a massive increase in research endeavours.
Acknowledgments
We would like to express our thanks to Editors Professor Peter Petros and Vani Bardetta for their tremendous help in designing and modifying the article.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the International Society for Pelviperineology for the series “Integral Theory Paradigm” published in Annals of Translational Medicine. Peter Petros (Editor) and Vani Bardetta (Assistant Editor) served as the unpaid Guest Editors of the series. The article has undergone external peer review.
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-23-1770/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-23-1770/coif). The series “Integral Theory Paradigm” was commissioned by the International Society for Pelviperineology without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All clinical procedures described in this study were performed in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for the publication of this article and accompanying images. Human participation in the video was by patient permission on the basis it was deidentified.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Petros PE, Ulmsten U. An Integral Theory of female urinary incontinence. Acta Obstet Gynecol Scand 1990;69:7-31. [Crossref] [PubMed]
- Petros P, Quaghebeur J, Wyndaele JJ. Defining urge as an uncontrolled micturition explains pathogenesis, informs cure and helps solve the burgeoning OAB crisis. Neurourol Urodyn 2022;41:1281-92. [Crossref] [PubMed]
- Inoue H, Kohata Y, Fukuda T, et al. Repair of damaged ligaments with tissue fixation system minisling is sufficient to cure major prolapse in all three compartments: 5-year data. J Obstet Gynaecol Res 2017;43:1570-7. [Crossref] [PubMed]
- Petros PE, Swash M, Kakulas B. Stress urinary incontinence results from muscle weakness and ligamentous laxity in the pelvic floor. Pelviperineology 2008;27:107-9.
- Petros PE, Ulmsten U. Bladder instability in women: a premature activation of the micturition reflex. Neurourol Urodyn 1993;12:235-9. [Crossref] [PubMed]
- Papa Petros PE. Detrusor instability and low compliance may represent different levels of disturbance in peripheral feedback control of the micturition reflex. Neurourol Urodyn 1999;18:81-91. [Crossref] [PubMed]
- Bush MB, Moron C, Messner-Pellenc L, et al. A mechanical model for the opening of the human female urethra. In: Adlassnig KP, Bracale M. editors. Proceedings of Biomedical Engineering 2005. Austria: Acta Press; 2005:210-3.
- Quaghebeur J, Petros P, Wyndaele JJ, et al. Pelvic-floor function, dysfunction, and treatment. Eur J Obstet Gynecol Reprod Biol 2021;265:143-9. [Crossref] [PubMed]
- Shkarupa D, Zaytseva A, Kubin N, et al. Native tissue repair of cardinal/uterosacral ligaments cures overactive bladder and prolapse, but only in pre-menopausal women. Cent European J Urol 2021;74:372-8. [Crossref] [PubMed]
- Inoue H, Kohata Y, Sekiguchi Y, et al. The TFS minisling restores major pelvic organ prolapse and symptoms in aged Japanese women by repairing damaged suspensory ligaments: 12 - 48 month data. Pelviperineology 2015;34:79-83.
- Inoue H, Nakamura R, Sekiguchi Y, et al. Tissue Fixation System ligament repair cures major pelvic organ prolapse in ageing women with minimal complications - a 10-year Japanese experience in 960 women. Cent European J Urol 2021;74:552-62. [Crossref] [PubMed]
- Petros PE, Ulmsten UI, Papadimitriou J. The autogenic ligament procedure: a technique for planned formation of an artificial neo-ligament. Acta Obstet Gynecol Scand Suppl 1990;153:43-51. [Crossref] [PubMed]
- Petros P, Palma P. Conceptualizing stress urinary incontinence surgery beyond midurethral slings: Very early results from simplified ligament repair without tapes. Neurourol Urodyn 2023;42:383-8. [Crossref] [PubMed]
- Goeschen K, Müller-Funogea A, Petros P. Tethered vagina syndrome: cure of severe involuntary urinary loss by skin graft to the bladder neck area of vagina. Pelviperineology 2010;29:100-2.
- Piñango-Luna S, Level-Córdova L, Petros PE, et al. A low-cost artisan tension-free tape technique cures pelvic organ prolapse and stress urinary incontinence: Proof of concept. Cent European J Urol 2020;73:490-7. [Crossref] [PubMed]
- Goeschen K, Gold DM. Surgical cure of chronic pelvic pain, associated bladder and bowel symptoms by posterior sling in 198 patients validates the Pescatori Iceberg principle of pelvic symptom co-occurrence. Pelviperineology 2017;36:84-8.
- Petros PEP, Richardson PA. TFS posterior sling improves overactive bladder, pelvic pain and abnormal emptying, even with minor prolapse: A prospective urodynamic study. Pelviperineology 2010;29:52-5.
- Petros PE. New ambulatory surgical methods using an anatomical classification of urinary dysfunction improve stress, urge and abnormal emptying. Int Urogynecol J Pelvic Floor Dysfunct 1997;8:270-7. [Crossref] [PubMed]


