
Wide-bore polyester sutures may create sufficient collagen for cure of prolapse/incontinence: a work in progress
Highlight box
Key findings
• Wide-bore polyester sutures create new collagen for ligament repair.
What is known and what is new?
• Native ligament Fothergill-type repairs for prolapse, midurethral slings for stress urinary incontinence (SUI).
• Wide-bore polyester sutures create new collagen to reinforce weakened structural collagen in suspensory ligaments.
What is the implication?
• Initial results for direct pubourethral ligament repair for SUI, and prolapse, seem to give equivalent results to sling repairs. This is promising, but for now, is a “work in progress”.
What should change now?
• Postmenopausal women having prolapse surgery could benefit significantly with use of No. 2 polyester sutures to plicate damaged ligaments instead of dissolving sutures.
Introduction
The key points of the article are summarized in the video abstract (Video S1).
“Necessity is the mother of invention.”—proverb.
This article introduces a less invasive collagen-creating surgical method for pelvic organ prolapse (POP) and pelvic symptom repair: ligament repair by wide-bore polyester sutures. The catalyst was the ban on all mesh surgery for POP (including slings) by major regulatory jurisdictions.
These promising new methods aim to continue the surgical revolution which began with the publication in 1990 of two major discoveries by Petros and Ulmsten, which fitted Thomas Kuhn’s description of a scientific revolution, the Integral Theory of Female Urnary Incontinence, and a new surgical principle, the creation of artificial collagenous neoligaments (1,2). (See URL: https://obgyn.onlinelibrary.wiley.com/toc/16000412/1990/69/S153).
The main thrust of the Integral Theory (1) which we follow in its entirety, was that control of bladder function was not from the bladder itself, but from ligaments and muscles outside of the bladder; that stress and urge dysfunctions were both mainly caused by vaginal and/or ligament laxity, whose pathogenesis was mainly weakened collagen and elastin (1). The consequence of this was that both stress and urge were potentially curable surgically, by repairing the ligaments, and this was demonstrated by several prototype operations (1).
Part of the 1990 publication (1) was a new surgical principle, how to repair weakened ligament collagen, by creation of an “artificial collagenous neoligament” (Figure 1) (2). The tissue reaction to an implanted tape was harnessed to create new collagen around the implanted tape (3), which became the basis for the prototype (3) and definitive midurethral sling (MUS) operation (4).
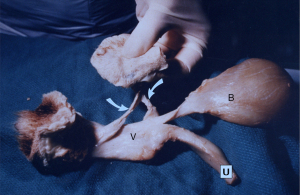
Evolution of collagen-creating slings
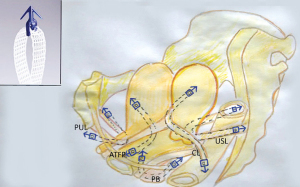
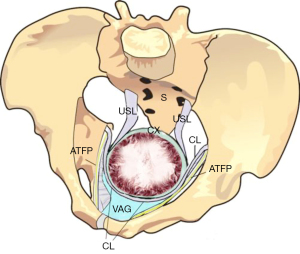
The protoype MUS (3) went on to become the “reverse vaginal tape procedure (TVT)” (4). The MUS has become the gold standard operation for cure of stress urinary incontinence (SUI), with 10 million operations by 2019 (5). In 2005, a single-incision minisling introduced a safer technique for neo-collagen creation (6). The Tissue Fixation System (TFS) minisling made it possible to repair all five main pelvic ligaments vaginally (Figure 2) (7). Inoue et al. confirmed the safety and efficacy of the minisling for cure of pelvic organ prolapse (POP) and lower urinary tract symptoms (LUTS) (7). Over a 10-year period, Inoue et al. implanted 3,100 TFS (Tissue Fixation System) minisling implants in 960 older women (mean age 70 years) (7). The hospital stay was day or overnight, with anatomical cure rates of 90% for all prolapses (7).
The 90% minisling prolapse results for slings (7) were in sharp contrast to the vaginal repair data from the Lancet Prospect Trial, whose cure rate at 12 months was in the region of 20% (8). Though achieving 85% cure for POP in premenopausal women using native CL/uterosacral ligament (CL/USL) repair, Shkarupa et al. (9), reported only 20.5% anatomical cure in postmenopausal women at 12 months. The poor results in postmenopausal women were attributed by Shkarupa et al. to the breakdown and excretion of collagen from the pelvic ligaments (9) after the menopause (10). Routine use of collagen-creation tapes in postmenopausal women was recommended (9).
Banning of slings for POP
In spite of their effectiveness and high cure rates (6,7), mesh sling operations, even the highly successful MUS, are among the most litigated operations in the history of surgery and have been banned in many countries. The medicolegal issues seem to be based on tissue reactions from tapes, yet that is how the MUS works (2). The catastrophically low results for “native tissue” repair (8) is evidence of a crisis, that, in some nations, postmenopausal women have few if any surgical options.
Slings versus native tissue
What Inoue et al. (7) demonstrated using the Tissue Fixation System (TFS) mini sling (Figure 2) was that collagen-creation tapes applied to all five pelvic ligaments could cure not only organ prolapse, but bladder, bowel, and pain symptoms also. Inoue et al.’s patients had a mean age of 70 years.
What Shkarupa et al. (9) and the Lancet Prospect Trial (8) demonstrated, was that postmenopausal women, who seemed to have the most severe symptoms and prolapses, could not be helped by native tissue repair, and required a technique which created new collagen to repair damaged ligaments.
The reality facing older women today who have POP or even SUI, is to have “native tissue repair” with its attendant poor cure rates or continue to suffer with their problem.
Wide-bore ligament plication—a possible solution?
The banning of slings raised the question, “Was there some other way to create new collagen for damaged ligaments?” A possible solution was found from re-examination of original data from the Doctor of Surgery Thesis by Peter Petros (11), where collagen from a rejected polyester aortic graft was tested for breaking strain on an Instron Tensiometer (11). It was calculated that the breaking strength of neocollagen created by No. 2 wide-bore polyester sutures (12), would be 2 orders of magnitude (×100) beyond the strength of a natural ligament (13). It could be predicted, therefore, that simply suturing any of the ligaments in Figure 2, with wide-bore No. 2 or No. 3 polyester sutures should create sufficient collagen to repair them, specifically, PUL for SUI cure and USL for uterine/apical prolapse cure. (See URL: https://doi.org/10.1002/nau.25049).
Unlike a sling which created a completely new collagenous neoligament, wide-bore No. 2 polyester sutures would work as well, only if the sutures had been accurately placed in the collagen-deficient part of the ligament. Normally this would be the proximal 5 cm of the ligament, the part nearest the pathway of the head as it descends down the birth canal.
The collegenopoietic principle developed in the experimental animals (2), indicated that the same ligament repair principle applied to reinforce pubourethral ligaments (PULs) for SUI (12), could also be effectively applied to repair damaged CLs for transverse defect cystocele, USLs for uterine/apical prolapse, and elongated deep transversus perinei (DTP) ligaments for cure of large perineoceles causing obstructive defecation and descending perineal syndrome (DPS). Such operations have now been performed (as yet unpublished data). Twelve-month results are favourable for all these conditions to date, albeit on a limited scale.
An important question for the new wide-bore polyester suture method is whether the early successes reported with these operations can continue in the longer term. The original canine experimental results (2) demonstrated that by 3 months the collagen formed was collagen 1, which has a breaking strength of 18,000 lbs/sq inch (14). It can be deduced from this, that, if the weak ligament was the only pathogenic factor, the cure of SUI or prolapse observed even by 6 months, should hold for the longer term. Nilsson et al. (15) demonstrated very little deterioration in the 12-month TVT operation cure rate 17 years postoperatively, and Inoue et al. reported similar long-term efficacy by repairing other ligaments, for example, cardinal, uterosacral, perineal body (PB) for cystocele, uterine prolapse and rectocele over a 10-year period (7).
Though they are both collagen creating methods there are important differences between a sling and wide-bore polyester sutures. Slings create totally new “artificial, collagenous neoligaments” (1). By placing a tape alongside the original ligament, for example, with a MUS for cure of SUI, a collagenous “U” is created below the urethra which attaches to the posterior surface of the pubic symphysis. A wide-bore polyester suture works differently. The tissue reaction around the sutures creates new collagen to add to the existing collagen in the ligament.
Methods and results for individual conditions
What is it for? Salvador Gil Vernet [1892–1987], famous Spanish anatomist/surgeon.
It is not possible to repair pelvic organ prolapse unless the surgeon first understands the contribution of each individual structure to the normal function of the system and its pathogenesis. Therefore, a short pathogenesis precedes the surgical descriptions.
The new pubourethral plication procedure [urethral ligament plication (ULP)] for cure of SUI
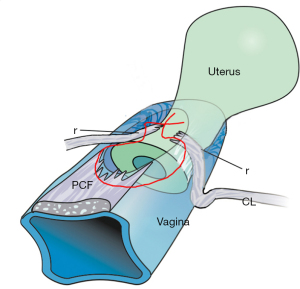
The pathogenesis of SUI shows that a weak PUL extends downwards on effort and fails to support the posterior urethral wall and vagina (Figure 3, broken lines) (12). The reflex posterior closure muscles, the levator plate (LP) and conjoint longitudinal muscles of the anus (LMA), pull down the trigone and bladder base as they do for bladder neck closure, but this has the effect of opening the urethra from “C” closed to “O” open. This forcible urethral opening exponentially lowers the urethral resistance to urine flow, and results in urine leakage on effort (SUI), sometimes even minimal effort.
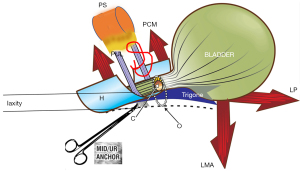
The ULP procedure is described in Figures 3,4, and Videos S2,S3. In essence, two parallel full thickness incisions are made from the bladder neck to the external meatus to expose both branches of the PUL (Figure 4). A No. 2 or No. 3 polyester suture binds the PUL to stop the PUL extension seen on effort on ultrasound. (See URL: https://doi.org/10.1002/nau.25049).
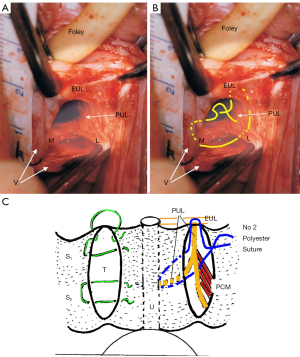
Initial results using wide-bore collagenopoietic polyester sutures for SUI, cystocele, and uterine prolapse, repairing ligaments only, are impressive, with 83% cure at 12 months (as yet unpublished data) , including the learning curve, estimated at about 5-6 cases for each surgeon.
Pathogenesis of cystocele and uterine/apical prolapse
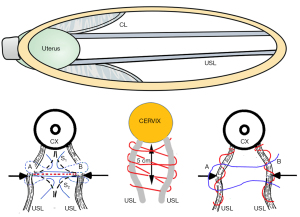
With reference to Figure 5, the cervix is at full dilatation, 10 cm. The collagen bonds of CL and USL have been depolymerized and have lost 90% of their strength, so the ligaments have become plasticized. The head stretches the USLs and CLs in the 5 cm where they attach to the cervix, and it is this part of the ligaments which must be repaired by No. 2 polyester sutures.
The pathogenesis of transverse defect cystocele is graphically represented by Figures 5,6. With reference to Figure 5, the pubocervical fascia (PCF) of the vagina attaches to CL, where the CL extends its attachment onto the anterior wall of the cervix. Figure 6 shows how a damaged CL prolapses lateral to the cervix or hysterectomy scar. Figure 6 also shows how the torn PCF leaves a fascial gap, so the bladder presses down on a vaginal epithelium unsupported by CL and PCF fascia like a trapdoor, to form a cystocele.
Note: the CL is damaged in all cystoceles and so needs to be repaired. In 80% of cystoceles, this is sufficient. In another 20%, the damage is more extensive and there remains a distal bulge of shiny epithelium, a “central” cystocele. The seemingly excess vagina should not be excised. The epithelium needs to be re-assigned as in Figures 6-8.
Surgical repair of CL for cure of cystocele
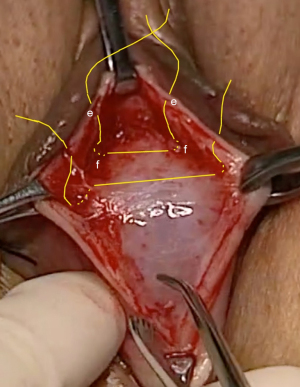
For repair of cystocele by a horizontal incision, a full thickness 5 cm horizontal incision is made at the junction of the bladder to the cervix. The bladder is dissected off the cervix. The proximal parts of the displaced CLs are approximated and sutured onto the anterior wall of the cervix with No. 2 polyester sutures. The advantage of a horizontal incision is that it makes the displaced CL easier to find, as the edge of the incision is sited just above the site of the displaced CL. The disadvantage is a second, longitudinal incision has to be made between the CL and the bladder neck if there is a shiny central defect which needs to be repaired. (See Video S4 CL repair transverse incision: Surgeons Ray Hodgson, Peter Petros).
For repair of cystocele by a longitudinal incision, a full thickness incision is made from the cervix to just short of the bladder neck area of the vagina. The bladder is dissected off the cervix. The proximal parts of the displaced CLs are approximated and sutured onto the anterior wall of the cervix with No. 2 polyester sutures. If there is a central cystocele distal to the CL repair, it is important not to excise any vaginal tissue. It can be re-assigned by following the technique in Figure 8.
The advantage of a longitudinal incision is that it can also repair a distal (“central”) cystocele through the same incision. The Video S5 by Professor Xiuli Sun repairs both cystocele and uterine prolapse with longitudinal incisions.
Uterine/apical repair
With reference to Figure 5, the head may stretch apart the USLs to cause an enterocele of varying width. If the collagen is damaged sufficiently, the USLs cannot support the uterus, and it will prolapse downwards, as in Figure 7A. Note: as the uterus prolapses down to the introitus, the CLs also invariably lengthen, which demonstrates why both USL and CL need to be repaired in women with major uterine apical prolapse.
A transverse incision is a very simple and a quick way to repair the USLs with excellent results, especially for women who have minimal prolapse (up to 2nd degree) and major LUTS symptoms. A 5 cm long full thickness transverse vaginal incision is made at the apex of the bulge in the posterior vaginal wall, usually about 4 cm down from the cervix. The enterocele is not entered. It is gently pushed away. The USLs are usually laterally displaced. The advantage of a transverse incision is that even if the USLs cannot be located, a suture placed 1 cm below the vaginal skin will invariably penetrate the USL. Two sutures as in Figure 7B (left) are usually sufficient if prolapse is minimal. (See Video S6 USL transverse incision by Surgeons Ray Hodgson and Peter Petros).
In women with major uterine prolapse (3rd & 4th degree), a longitudinal incision is indicated. First the prolapse is reduced. It is necessary to restore the uterus to its anatomical position, so as to facilitate the ligament repair. The enterocele is not entered. It is gently pushed away. The USLs are usually laterally displaced, so they need to be identified and plicated with No. 2 or No. 3 polyester sutures (Figure 7B, middle frame). If the ligaments are very thin, they need to be identified and plicated individually with No. 2 or No. 3 polyester suture (Figure 7B right frame), then approximated with one or two interrupted polyester sutures. The individual plication ensures the USLs are reinforced by the collagen produced by the polyester sutures.
Excess vaginal tissue is dealt with using the “concertina suture” (Figure 8).
No. 2 polyester repair of perineocele and DPS
Understanding the pathogenesis of DPS, that the descending head stretches, elongates and pushes the PB structures laterally, gives important insights for the surgery. With reference to Figure 9, the outlet of the pelvis is 12–13 cm wide. The PB is a 4 cm fibromuscular body composed mainly of collagen, elastin, smooth muscle, and some striated muscle separates the vagina from the rectum. The PB is suspended by the DTP ligaments, which are 4 cm long and which insert behind the upper 2/3 and lower1/3 of the descending ramus (Figure 10).
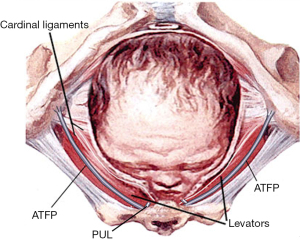
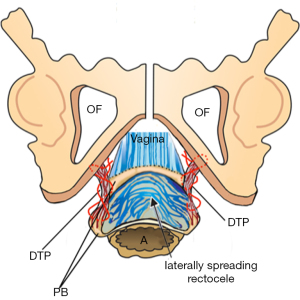
At delivery, every part of the outlet is stretched by the head, especially the PB and its suspensory ligaments, DTP. A fully flexed head has a diameter of 9.4 cm, and if deflexed, it is 11.2 cm, and will clearly cause more damage on the way out.
The surgery commences with a transverse incision just inside the hymen. Alternatively, a full thickness longitudinal incision can be made from the hymen to the vaginal apex. Often, there is a virtual absence of the PB with only a very thin (1 mm thickness) rectal mucosa adherent to a layer of thin vaginal epithelium, spread laterally, densely adherent to the PB and vaginal wall, as in Figure 10. A finger in the rectum is essential to identify the spread of the rectal mucosa. This has to be done with care, as the mucosa is only 1 mm thick and easily perforated. If a perforation occurs, it can be easily restored with 2-layer repair. The rectal mucosa is very carefully dissected off the vagina, PB and if it is adherent to it, the descending ramus also. The smooth muscle layer of the rectum is usually split and retracted laterally. It is restored with 2–3 vicryl sutures.
The PB is firm, whitish in appearance, laterally displaced, and sited below the ischial tuberosities. Once the PB is dissected fully from the adherent rectum, it is firmly grasped with forceps. A No. 1 vicryl holding suture is inserted to lift the PB up into the operating field. (See Video S7, dissection and Video S8, surgery).
Identification of the DTP ligament required dissection of the rectum off the vagina and laterally displaced PBs. The Vicryl sutures identifying PB, are stretched towards the operator, and the surgeon’s index finger in the rectum identifies the insertion of the DTP into the posterior part of the lower third of the descending ramus.
A No. 2 polyester suture attached to a firm 1.25 cm needle shortens and reinforces the DTP. The first suture is applied immediately behind the descending ramus, brought down to the PB held by the No 1 vicryl suture, and then brought back up to be tied, as in Figure 10, but not tightly. The DTP repair is repeated contralaterally. The superficial transverse muscles and the vagina are gently approximated.
This operation needs to be viewed in the context of the strong definitive statement by Chaudhry and Tarnay (16), “It is controversial whether surgical management is even an option for patients with DPS” (16).
Deep transverse perinei ligament repair for DPS is a very promising but still emerging operation with only limited numbers (n=4), to date. Encouragingly, all were cured at 12 months review, not only of their DPS but clinical symptoms such as urge, fecal incontinence, obstructive defecation, dragging pain and hemorrhoids.
Conclusions
Except for the ULP operation for cure of SUI, the techniques for cystocele; uterine prolapse perineocele were essentially evolved versions of the Fothergill and standard PB repairs without any vaginal or ligament excisions. Though promising, more extensive, and longer-term results are clearly required before these methods can become mainstream.
Acknowledgments
We would like to express our gratitude to Editors Professor Peter Petros and Vani Bardetta for their exceptional support in the design and refinement of the article.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the International Society for Pelviperineology for the series “Integral Theory Paradigm” published in Annals of Translational Medicine. Peter Petros (Editor) and Vani Bardetta (Assistant Editor) served as the unpaid Guest Editors of the series. The article has undergone external peer review.
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-23-1774/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-23-1774/coif). The series “Integral Theory Paradigm” was commissioned by the International Society for Pelviperineology without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All clinical procedures described in this study were performed in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for the publication of this article and accompanying images and videos. Human participation in the videos was by patient permission on the basis it was deidentified.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Petros PE, Ulmsten U. An Integral Theory of female urinary incontinence. Acta Ob-stet Gynecol Scan 1990;69:7-31. [Crossref] [PubMed]
- Petros PE, Ulmsten U, Papadimitriou J. The Autogenic Neoligament procedure: A technique for planned formation of an artificial neo-ligament. Acta Obstet Gynecol Scand 1990;69:43-51. [Crossref] [PubMed]
- Petros PE, Ulmsten U. The combined intravaginal sling and tuck operation: An ambulatory procedure for stress and urge incontinence. Acta Obstet Gynecol Scand 1990;69:53-9. [Crossref] [PubMed]
- Ulmsten U, Petros P. Intravaginal slingplasty (IVS): An ambulatory surgical procedure for treatment of female urinary incontinence. Scand J Urol Nephrol 1995;29:75-82. [Crossref] [PubMed]
- Toozs-Hobson P, Cardozo L, Hillard T. Managing pain after synthetic mesh implants in pelvic surgery. Eur J Obstet Gynecol Reprod Biol 2019;234:49-52. [Crossref] [PubMed]
- Petros PEP, Richardson PA. The midurethral Tissue Fixation System sling: A ‘micromethod’ for cure of stress incontinence- preliminary report. ANZOG 2005;45:372-5. [Crossref] [PubMed]
- Inoue H, Nakamura R, Sekiguchi Y, et al. Tissue Fixation System ligament repair cures major pelvic organ prolapse in ageing women with minimal complications: A 10-year Japanese experience in 960 women. Cent European J Urol 2021;74:552-62. [Crossref] [PubMed]
- Glazener CMA, Breeman S, Elders A, et al. Mesh, graft, or standard repair for women having primary transvaginal anterior or posterior compartment prolapse surgery: Two parallel-group, multicentre, randomised, controlled trials (Prospect). Lancet 2017;389:381-92.
- Shkarupa D, Zaytseva A, Kubin N, et al. Native tissue repair of cardinal/uterosacral ligaments cures overactive bladder and prolapse, but only in pre-menopausal women. Cent European J Urol 2021;74:372-8. [Crossref] [PubMed]
- Sone T, Miyake M, Takeda N, et al. Urinary excretion of type I collagen crosslinked N-telopeptides in Healthy Japanese adults: Age- and sex-related changes and reference limits. Bone 1995;17:335-9. [Crossref] [PubMed]
- Petros PE. Development of the Intravaginal Slingplasty and other ambulatory vaginal operations [Doctor of Surgery Thesis]. Perth (WA): University of Western Australia; 1999.
- Petros P, Palma P. Conceptualizing stress urinary incontinence surgery beyond midurethral slings: Very early results from simplified ligament repair without tapes. Neurourol Urodyn 2023;42:383-8. [Crossref] [PubMed]
- Yamada H. Aging rate for the strength of human organs and tissues. In: Evans FG editor. Strength of biological materials. Baltimore: Williams & Wilkins Co; 1970. p. 272-80.
- Florey H. General Pathology. Lloyd Luke; 1971.
- Nilsson CG, Palva K, Aarnio R, et al. Seventeen years' follow-up of the tension-free vaginal tape procedure for female stress urinary incontinence. Int Urogynecol J 2013;24:1265-9. [Crossref] [PubMed]
- Chaudhry Z, Tarnay C. Descending perineum syndrome: a review of the presentation, diagnosis, and management. Int Urogynecol J 2016;27:1149-56. [Crossref] [PubMed]


