
Tethered vagina syndrome: massive urine loss caused by bladder neck scarring cured by skin graft
Highlight box
Key findings
• Tissue scarring in the bladder neck area of vaginal “zone of critical elasticity” (ZCE), whether iatrogenic or obstetric fistula induced, can cause massive urine loss.
What is known and what is new?
• Tethered vagina syndrome (TVS) is not generally known. “Stovepipe urethra” may be similar.
• TVS is a newly described condition. Scarring at ZCE links muscle forces to forcibly open the urethra to cause massive urine loss. Cure is by a skin graft to ZCE to restore elasticity.
What is the implication and what should change now?
• Understanding vaginal elasticity is essential for normal bladder function.
• Avoiding tissue excision during vaginal repairs.
• Consider preventative skin graft during obstetric fistula surgery.
Introduction
The key points of the article are summarized in the video abstract (Video S1).
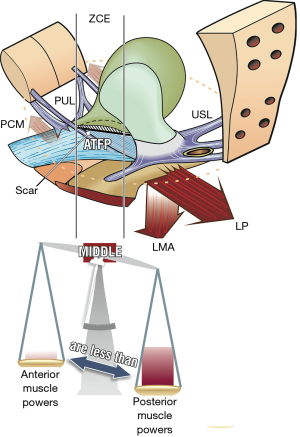
Tethered vagina syndrome (TVS) as first reported in the early 1990s, was described as an iatrogenic condition (1-3). The classic TVS symptom is massive uncontrollable urine loss immediately the patient’s foot is placed on the floor on getting out of bed in the morning, usually without urgency. Another symptom is sudden leaking on getting off a chair. Stress urinary incontinence (SUI) is minimal or absent. The cause is scar tissue across an elastic area across the bladder neck of the vagina, known as the “zone of critical elasticity” (ZCE) (1) (Figure 1). The scar prevents the separate and independent function of the distal urethral and bladder neck closure mechanisms which are operated by opposing pelvic muscle forces. With reference to Figure 1, the scar “tethers” the more powerful posterior muscle forces, the levator plate and conjoint longitudinal muscle of the anus (LP/LMA) to the weaker anterior muscle forces, the pubococcygeus muscle (PCM), and forcibly overcomes them to open the outflow tract exactly as happens during micturition.
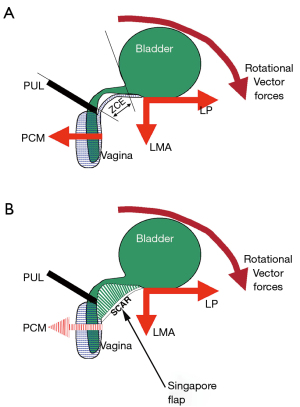
There are two distinct phenotypes of TVS:
- Iatrogenic scarring (developed countries);
- Obstetric fistula scarring (developing countries).
Links to TVS teaching modules and for post-fistula repair leakage:
- https://www.pelviperineology.org/pdfs/PPJ_39_2_38_39.pdf;
- https://pelviperineology.org/pdfs/PPJ_39_2_42_49.pdf;
- https://cms.galenos.com.tr/Uploads/Article_36941/Pelviperineology-34-48-En.pdf;
- https://www.pelviperineology.org/pdfs/PPJ_36_1_11_12.pdf.
Developed countries (iatrogenic scarring)
TVS is usually caused by scar tissue in the bladder neck area of the vagina from “native tissue vaginal repair”, but it may be caused by mesh sheets inserted for cystocele repair, or excessive bladder neck elevation from a Burch colposuspension (1-6). Another cause, for slightly different reasons, is by a transobturator (TOT) sling where the TOT is misplaced into the lateral part of PCM which contracts backwards on effort to open the urethra.
Diagnosis
TVS can be confirmed during vaginal examination where a scar is always present at the bladder neck (Figure 2). Usually, there is no urine loss on coughing. Typically, applying Allis forceps gently behind the urethrovesical junction and stretching the vagina backwards may cause urine leakage. This confirms TVS. Transperineal 2D ultrasound (Figure 3) is helpful: failure of the bladder neck to descend on straining is an important sign.
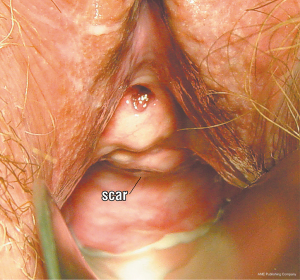
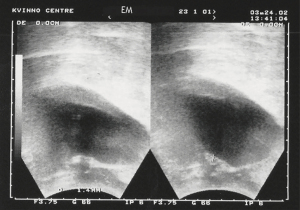
Pathogenesis
Scarring at ZCE tethers the forward closure needed to close the distal urethra to the more powerful backward muscles which now forcibly open the urethra on effort. Hence the name, “tethered vagina”.
Surgical repair
This begins with the surgical release of the vagina from its scarred attachments to the urethra, bladder and pubic bones. The aim is to restore the elasticity in the bladder neck area of the vagina (ZCE) so the two separate closure mechanisms can operate independently of each other. If, after the anatomy has been restored by full dissection, there is no gap between the dissected walls of vagina, it is reasonable to assume that there is no tissue deficit (this is usually the case after TVS from Burch colposuspension). In such cases, an “I-plasty” can be performed; I-plasty involves wide dissection of the vagina off the bladder and urethra. The cut vaginal ends are then approximated horizontally.
Indications for a graft
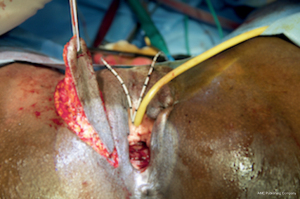
The cut ends must fall together naturally. If they do not, a skin graft must be applied to restore elasticity. Failing this, the vaginal tissues will need to be stretched to cover the space and this will result in surgical failure within twelve weeks with more scar formation. A direct graft using lower abdominal skin can work well. The fat from the skin is removed. Extreme care needs to be taken to suture a direct graft onto the base so as to avoid hematoma, which will cause failure. It is important to ensure that there is good hemostasis where any skin graft is to be applied. A split labium minus graft (6,7) works very well, as does a skin-on Martius graft (3,4,8) Figure 4), and Singapore flaps (9,10), as these carry their own blood supply.
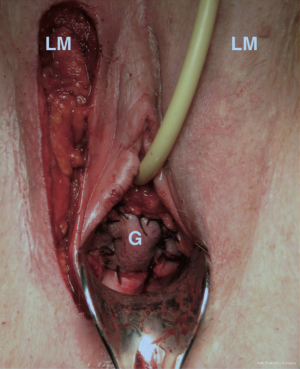
Which graft operation?
Whatever operation is applied needs to restore the elasticity of the ZCE. Each operation has its problems. Direct skin grafts from the lower abdomen must revascularize from the bladder base and adjacent tissues (2). The split labium minus graft carries its own blood supply and works well (7); however, it requires an adequately large labium minus, significant surgical skill, and may be esthetically displeasing for the woman as she is left with one labium minus. Skin-on Martius graft (4,5) and Singapore flap grafts (9-11) carry their own blood supply but must be passed through a channel between the vulval skin and bone to reach the vagina. Post-operative constriction of the channel is a concern as it can choke off the blood supply for the graft. A skin-on Martius graft often carries hairs into the vagina, and this may concern some women. Singapore flap grafts, being from the inner thigh, are hairless, and provide a more extensive graft for obstetric fistula patients.
Goeschen (4,5) reports significantly better results with a skin-on Martius graft technique, than with an I-plasty or free skin graft. He compared three methods for cure of the TVS (n=57): I-plasty (n=13); free skin graft (n=21); bulbocavernosus-muscle-fat-skin-flap from the labium majus, (n=23). At 6-month follow-up, cure rates were 23%, 52% and 78% (urine loss of <10 g/24 hours) (4).
Developing countries (obstetric fistula scarring)
The most dramatic application of the TVS has been in substantially solving the problem of ongoing massive urine loss after successful closure of obstetric fistula (8-10). In developing countries, up to 50% of women, who have had successful closure of their vesico-vaginal fistula, continue to have massive urinary leakage.
The initial hypothesis was that these women had TVS from tissue necrosis and consequent scarring in the bladder neck area of the vagina caused by pressure from the fetal head (7). The hypothesis was directly tested by surgical release of the vagina from its scarred attachments to the urethra and pubic bones (8-10). The protocol for a prophylactic graft was that if, after repair of the bladder defect, there was a gap between the dissected walls of the vagina (i.e., a tissue deficit), a skin graft was inserted to restore the elasticity required in the bladder neck area of the vagina (ZCE). Initially, a skin-on Martius graft was used to cover the defect (8). Subsequently, Dr. Browning found that a skin-on “Singapore flap”, a larger graft from skin from the inner thigh, was more appropriate, and he produced quite remarkable initial results (9-10), which have since been confirmed and even further improved. Used prophylactically with Goh type 4 fistula (n=45), 46% were dry against an expected 19%. In patients with successful fistula closure, still with severe leakage (n=24), 71% were dry against an expected 26%.
A new classification
We believe that there is only one issue as regards fistula classification. If, after full dissection of the vagina off the pubic bones and urethra, the two sides of the vagina remain separated, then a skin-on flap is required. Any forcible approximation of tissue may lead to scarring and surgical failure.
Typical clinical presentation
A typical clinical presentation of surgery-induced TVS usually involves postmenopausal women with multiple previous operations for prolapse some years earlier. Often, they have also had a Burch colposuspension for incontinence. More recently, TVS has been appearing in women with large mesh sheets implanted in their vagina. On specific questioning, almost all admit to the cardinal symptom of this condition, massive uncontrolled bladder emptying immediately when their foot touches the floor on getting out of bed in the morning. Another common symptom is losing urine on standing up from a chair or when bending down.
A large amount of urine measured by 24-hour pad tests attest to the seriousness of this problem. On examination, urine loss during coughing is not a common feature of this condition. There is very little movement of the bladder neck during straining with ultrasound testing, consistent with the thick scarring observed in the bladder neck area of her vagina. However, gently stretching the vagina behind the bladder neck backwards with Allis forceps usually elicits a gush of urine on coughing in such patients.
How TVS causes massive urine loss
The “TVS” is still not a well-recognized condition. In developed nations, it is entirely iatrogenic, and is caused by excessive scarring from previous surgeries. Simplistically, with reference to Figure 5, the dense scar tissue in the vagina “tethers” the backward-acting opening muscles (LP/LMA) to the forward acting closure muscles (PCM), so the posterior wall of the urethra is forcibly opened out, much as happens during micturition (12). This condition was not previously recognized as originating from a scarred vagina. It was thought to originate from the bladder itself, and was treated with drugs, which, of course, cannot succeed, as the cause is mechanical. Many women with TVS have been treated with a midurethral sling which, of course, worsens the condition as it adds more scar tissues to the area. Treatment involves restoration of elasticity in the bladder neck area of the vagina, using some type of skin graft. Restoration of continence following skin graft surgery is the ultimate proof of the role of the three reflex muscle forces in bladder function (and dysfunction) (Figures 1,5).
Why there is little or no urine loss on coughing in TVS
Coughing involves fast-twitch muscle action. Urine is not lost during the time of the cough because the backward muscle forces do not have enough time to stretch the scar sufficiently to forcibly overcome the forward closure effect by the PCM. Urine loss on getting out of bed in the morning requires prolonged, strong, slow-twitch pelvic muscle contraction to stabilize the pelvis prior to the thigh, abdominal and back muscles lifting the torso to get out of bed. The prolonged contraction of the posterior muscles, forcibly overcomes the PCM closure of the urethra, and urine floods out uncontrollably.
Towards a solution of obstetric fistula problems
Bladder damage from obstetric fistula is a major problem in developing countries such as Africa with at least 2 million women affected. Dr. Andrew Browning is an acknowledged fistula surgeon who has introduced a major advance in fistula surgery to solve the problem of women, who despite successful fistula closure, continue to lose massive amounts of urine afterwards. Dr. Browning is applying the principles of the TVS to restore elasticity to the bladder neck area of the vagina both pre-operatively and also in patients afflicted with this condition despite their successful fistula closure. We are reprinting an editorial by Dr. Andrew Browning by permission of the Pelviperineology journal (13).
I thank the editors of Pelviperineology for this invitation to write an editorial as background to our Interim Report (10), on a new method for fistula surgery, which was first proposed in this journal in 2015. The background is that despite the efforts of the Millennium Development Goals and recent advances in maternal health across the world, far too many women are still dying in labour and many women are still getting injured. The obstetric fistula is one of the most feared injuries and there are an estimated 2 million women across the world still waiting for treatment. There is a growing number of surgeons being trained and more women getting treated. But the surgery is not a guarantee of cure. Fistula patients vary considerably in the type of injuries they sustain and what type of surgery is needed to reconstruct the urinary tract, reproductive tract and gastrointestinal tract. Closing the defects is one thing and over 95% of women can have their fistula closed at the first operation by a skilled surgeon. However, up to 55% of patients will still have ongoing incontinence. This problem of ongoing incontinence has been underreported and even neglected. Many places just perform a dye test and if it is negative the patients are recorded as cured, even if they are still leaking the same amount as before the operation. The harder you look for the problem, the more you will find. It is the author’s routine to examine all patients with a full bladder, get them to cough, walk and for the more severe cases quantify the loss with a 1-hour pad test. Accurate diagnosis is of critical importance. Those patients who remain wet become depressed, suicidal with little or no chance of having a normal life. Many, very many, live out their lives as outcasts from family and home. There have been several different operations described to tackle the problem. The general principles of urinary incontinence in the west are not readily transferable as the pathology is different. The patients with ongoing incontinence invariably have had some damage of their urethra during the fistula formation, sometimes the whole urethra along with half the bladder has been destroyed along with the vagina and despite complex reconstructive surgery, they are still wet. Some have tried tape slings, but with poor results and high erosion rates. Nearly all hospitals performing fistula surgery cannot afford synthetic slings anyway. Autologous slings, muscle or fascia are cheap and so are used more widely, but it is not the answer. For the most severe cases the cure rate is a pitiful 26%. Our introduction of a skin flap to restore vaginal elasticity has shown a big jump in the success rates in women that would have been labeled as having an extremely poor prognosis or even inoperable. We could expect even greater outcomes in all patients with vaginal tissue loss. This Interim Report is the endpoint of a classic scientific journey. Guided by the Integral Theory, an analysis was made of the pathogenesis; a hypothesis was formed, that the problem was scar-induced loss of vaginal elasticity. From this evolved treatment, application of a skin graft to improve tissue elasticity. This was tested in a small pilot study. Then the skin graft method was applied to the worst affected cases, the basis of our Interim Report. In summary, this technique marks the most exciting advancement in fistula surgery for many years and many thousands of impoverished women stand to benefit.
Singapore flap used prophylactically
The first series of patients who had the Singapore flap had the most severe obstetric fistulas (10,11). They were Goh type 4ciii, large (>3 cm but up to 10 cm in diameter), scarred with often little to no vaginal tissue remaining and often circumferential.
It is possible to close the defect in such patients, with rates of 95% closure at the first operation recorded, but ongoing incontinence rates were very high, up to and exceeding 80%. With the introduction of the Singapore flap the ongoing incontinence rates decreased to 54%. With severe Goh type 3 fistula (which have a marginally better prognosis than Goh type 4), the ongoing incontinence rates without the flap were 54% and with the introduction of the flap, it reduced to 13%.
Singapore flap used after successful closure and continued leakage (see Video S2)
Patients often came back after fistula closure for management of their ongoing incontinence. Fistula surgeons have been achieving good results when focusing on making the urethra the normal length and width and by reconstructing the pubourethral ligament. However, the introduction of the Singapore flap to scarred, tethered vaginas (Figure 6) increased the success rate dramatically (9-11). It was first used on patients who had failed all previous attempts at repair; some had been operated on up to nine times at various hospitals and were still completely wet with an average 1-hour pad test of 224 mL. Dissecting and releasing the tension on the anterior vaginal wall would make the vagina literally “spring” open. The urethral meatus would spring forward, and the cervix could be pushed back. A 2–3 cm gap in the vagina would be created and filled with the Singapore flap; 71% of these patients became completely continent and most of the remaining patients improved. In contrast, similar cases operated on without the flap and paying attention to the tethered anterior vagina resulted in only 26% who became dry.
Conclusions
In the iatrogenic phenotype, the overwhelming challenge is to recognize large amounts of urine loss on specific types of effort as a pathogenic condition. TVS is not, at present, well known. Despite little or no loss on coughing, many surgeons perform a midurethral sling, which worsens the problem.
The understanding behind the mechanism of TVS is not only an important component in the successful management of this condition but also acts as a proof positive for the integral theory generally. The fact that the urethra is maintained in an open position by the scar tissue confirms the presence of the posterior forces that ordinarily act during micturition to open the bladder neck/urethra and explains why the patient suffers from ongoing leakage. Consequently, the formation of further collagen and scar tissue with the misguided placement of a midurethral sling fails to address these symptoms and will frequently make them worse. Normal function can only be returned by procedures that aim to restore the normal functional elasticity in this area; that is, the ZCE, adding further evidence to its existence. For the fistula phenotype, a Singapore flap operation is proving to be outstandingly successful.
Acknowledgments
We would like to express our gratitude to Editors Professor Peter Petros and Vani Bardetta for their exceptional support in the design and refinement of the article.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the International Society for Pelviperineology for the series “Integral Theory Paradigm” published in Annals of Translational Medicine. Peter Petros (Editor) and Vani Bardetta (Assistant Editor) served as the unpaid Guest Editors of the series. The article has undergone external peer review.
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-23-1866/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-23-1866/coif). The series “Integral Theory Paradigm” was commissioned by the International Society for Pelviperineology without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All clinical procedures described in this study were performed in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for the publication of this article and accompanying images and videos. Human participation in the videos was by patient permission on the basis it was deidentified.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Petros PE, Ulmsten UI. The tethered vagina syndrome, post surgical incontinence and I-plasty operation for cure. Acta Obstet Gynecol Scand Suppl 1990;153:63-7. [Crossref] [PubMed]
- Petros PE, Ulmsten U. The free graft procedure for cure of the tethered vagina syndrome. Scand J Urol Nephrol Suppl 1993;85-7.
- Petros P. Chapter 4 Reconstructive pelvic floor surgery according to the Integral Theory textbook: the female pelvic floor, function, dysfunction and management, according to the Integral Theory. Heidelberg: Springer; 2010:179-87.
- Goeschen K, Müller-Funogea A, Petros P. Tethered vagina syndrome: cure of severe involuntary urinary loss by skin graft to the bladder neck area of vagina. Pelviperineology 2010;29:100-2.
- Goeschen K. Tethered vagina teaching module. Pelviperineology 2020;39:42-9.
- Petros P, Gunnemann A, Liedl B. Use of Martius flaps in complex female urethral surgery and the tethered vagina syndrome. Cent European J Urol 2014;67:208-9. [Crossref] [PubMed]
- Petros P. The split labium minus flap graft technique. Int Urogynecol J Pelvic Floor Dysfunct 2004;15:95-8; discussion 98. [Crossref] [PubMed]
- Petros P, Williams G, Browning A. Post vesico-vaginal fistula repair incontinence - A newhypothesis and classification potentially guide prevention and cure. Pelviperineology 2015;34:48-50.
- Williams G, Browning A, Petros PE. The integral theory and its tethered vagina syndrome revisited: vaginal scarring may cause massive urinary incontinence. BJU Int 2018;122:532-4. [Crossref] [PubMed]
- Browning A, Williams G, Petros P. Prevention and cure of post vesico-vaginal fistula repair incontinence by insertion of skin graft in the bladder neck area of vagina: Update on hypothesis and interim report. Pelviperineology 2017;36:9-11.
- Browning A, Williams G, Petros P. Skin flap vaginal augmentation helps prevent and cure post obstetric fistula repair urine leakage: a critical anatomical analysis. BJOG 2018;125:745-9. [Crossref] [PubMed]
- Petros PE, Ulmsten UI. An Integral Theory of female urinary incontinence. Acta Obstet Gynecol Scand 1990;69:7-31. [Crossref] [PubMed]
- Browning A. Editorial. Towards a solution for obstetric fistula problems. Pelviperineology 2017;36:2.


