
A practical ligament-based diagnostic system for cure of pelvic symptoms and prolapse
Highlight box
Key findings
• A practical diagnostic system which diagnoses ligamentous causation of bladder/bowel/pain symptoms.
What is known and what is new?
• Many bladder/bowel/pain symptoms are said to have unknown pathogenesis and to be, in the main, incurable.
• A holistic, comprehensive, step by step practical method for locating and guiding the repair of ligaments to cure or improve bladder/bowel/pain symptoms.
What is the implication, and what should we change now?
• The algorithm indicates which ligaments may cause bladder/bowel/pain symptoms, which can be confirmed by simple outpatient tests.
• Adoption of the diagnostic algorithm, as a standard assessment tool in women with bladder/bowel/pain symptoms, accurately guides ligament causation and helps guide decisions for surgery.
Introduction
The key points of the article are summarized in the video abstract (Videos S1-S3).
“It is not sufficient to describe an anatomical structure. An answer is required to the question, what is it for.”—Salvador Gil-Vernet (1892–1987) Famous Spanish Anatomist and Urologist and Nobel Prize nominee.
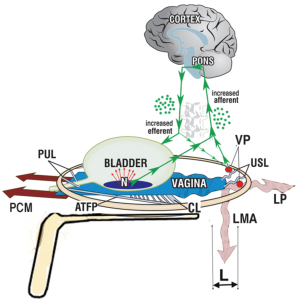
The Diagnostic System of the Integral Theory Paradigm (ITP) (1,2) is a practical guide designed for everyday clinical use (Figure 1). It is structured, anatomical, and symptom-based.
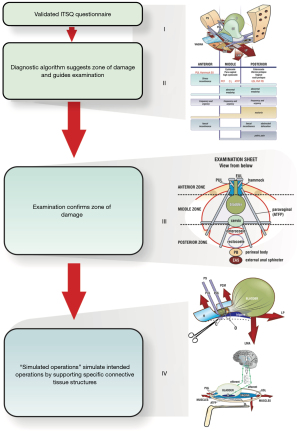
Why the ITP structured diagnostic system is different?
The ITP flow chart (Figure 1), is the only diagnostic system for diagnosing the anatomical causation of bladder/bowel/pain symptoms in existence. It is a 4-step comprehensive diagnostic system.
Why is a structured diagnostic system necessary?
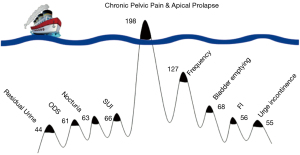
Simply stated, patients complain of only one main problem, the “tip of the iceberg” (3) (Figure 2). Only a structured questionnaire can identify all patient symptoms. In Figure 2, the main complaint in 198 women (3) was chronic pelvic pain (CPP). The other pelvic symptoms were “under the surface” and were diagnosed by the use of the Integral Theory Symptom Questionnaire (ITSQ) (4). The structured diagnostic system (Figure 1 flow chart), located many other bladder/bowel/pain symptoms which were present “below the surface”, but were not volunteered by the patients.
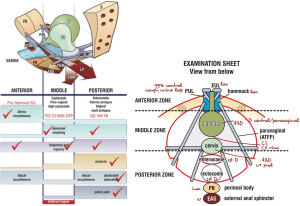
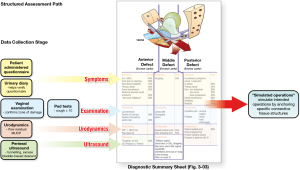
The structured assessment diagnostic system is summarized in a diagnostic flow chart
It is a comprehensive clinical assessment, recording and testing tool to determine ligamentous causes of pelvic symptoms and prolapse.
Figure 1 comprises 4 parts: (I) the validated ITSQ, locates symptoms; for the (II) diagnostic algorithm for initial diagnosis of ligament causation; which guides the (III) vaginal examination to confirm specific prolapses and ligament damage; which guides performance of (IV) “simulated operations” to confirm ligament pathogenesis: hemostat test to (i) relieve urine loss on coughing, speculum test to relieve urge and chronic; (ii) CPP, which more securely guides surgical or nonsurgical treatment.
Part I of the flow chart—the validated patient questionnaire (ITSQ)
With reference to the diagnostic flow chart (Figure 1), the Structured Assessment System begins with a completed questionnaire (4) (Figure 3) which uses an actual clinical case as an example: a parous woman with symptoms of stress urinary incontinence (SUI), overactive bladder (OAB) (urge, frequency, nocturia), abnormal emptying, CPP, and 4th degree cystocele and uterine prolapse.

Please note: the same clinical case example in the questionnaire [1], is used for the Algorithm [2] and for the examination sheet [3]. The results from pubourethral ligament (PUL) and uterosacral ligament (USL) surgery are written in red in the questionnaire.
How to use the ITSQ
The ITSQ (Figure 3) is a validated questionnaire and was designed to be self-administered (4). However, experienced clinicians advise more clarity of the answers can be achieved by direct questioning by the physician. “Sometimes” answers are ticked in the algorithm columns, even when incidence is low.
A,M,P (Anterior, Middle, Posterior), on the left side of the questionnaire, indicate zones of connective tissue (ligament) looseness. The appropriate columns, A,M,P, are ticked in the algorithm (see marked example, Figure 4). “TVS” on the left side signifies the question relates to the Tethered Vagina Syndrome (TVS), a condition caused by scarring or tightness in the bladder neck area of the vagina (middle zone), usually iatrogenic.
The diagnosis of ligament looseness and prolapse is then visually read off from the algorithm (see marked example, Figure 4).
The numbers 1–11 in the left column of the questionnaire explain the anatomical basis of the questions. This part is for the physician only and is located at the end of each questionnaire (see Appendix 1). The red writing shows the post-operative symptom results from midurethral and uterosacral sling operations.
Part II of the flow chart—the diagnostic algorithm
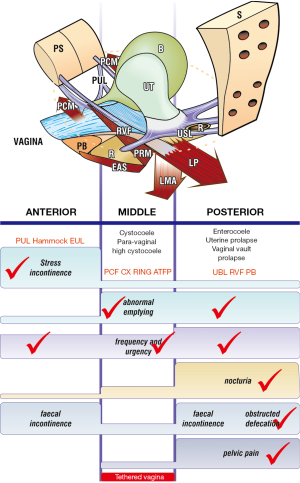
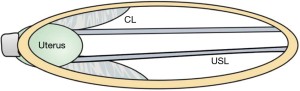
With reference to the diagnostic flow chart (Figure 1), answers from the questionnaire have been marked in each of the three columns (zones) of the algorithm (Figure 4). The algorithm indicates that the patient has four potentially damaged ligaments, PUL, cardinal ligament (CL), USL, perineal body (PB), and potential prolapses in all three zones:
- Anterior zone: suburethral vaginal hammock and external urethral ligament (EUL);
- Middle zone: cystocele;
- Posterior zone: uterine/apical prolapse and enterocele.
A diagnosis of ligament causation of symptoms and prolapse is indicative of pathogenesis, but it has to be checked by vaginal examination and “simulated operations” (Figure 1 flow chart).
Adoption of the diagnostic algorithm as a standard assessment tool in women with bladder/bowel/pain symptoms accurately guides ligament causation and helps guide decisions for surgery (see Appendix 2 for a clear copy of the diagnostic algorithm).
The algorithm used as a stand alone diagnostic tool
The diagnostic algorithm (Figure 4) can be used by itself, as a simple stand-alone diagnostic tool as part of every vaginal operation. The clinician uses the algorithm as an “aide memoire” and asks the questions directly. The algorithm is sufficiently accurate from a diagnostic perspective, and it ensures that every symptom and every prolapse is searched and recorded (see Table 1).
Table 1
| Instructions |
| (I) Tick symptoms in every box where they occur, even if they occur only “sometimes” |
| (II) Visually read off the prolapse and connective tissue structures which are potentially causing the symptom |
| Conditions |
| Nocturia, chronic pelvis pain and ODS occur only with USL laxity |
| Note: this simplifies diagnosis of USL causation and repair thereof |
| SUI of major degree is always caused by PUL weakness |
| Note: a midurethral sling or repair will or improve SUI and 50% urge symptoms, if it is mixed incontinence |
| Some symptoms, for example, urge and frequency, abnormal emptying, occur in more than one column and so may have more than one single causation |
Adapted from Petros P (1). With permission from Peter Petros; retains ownership of the copyright. ODS, obstructive defecation syndrome; USL, uterosacral ligament; SUI, stress urinary incontinence; PUL, pubourethral ligament.
Part III of the flow chart: vaginal examination
This is a “how to” instruction of what the ligament defects and prolapses predicted by the algorithm in each zone look like. At the end of the vaginal examination, the clinician completes the examination sheet for each individual patient, where the clinician records the findings for all the three zones. The examination sheet records ligament defects and degree of prolapse. It also serves as an aide memoir to ensure all three zones are examined.
Figure 5 is the actually completed examination sheet record of the ligament defects and degree of prolapse found in each of the three zones of the patient’s ITSQ completed questionnaire (Figure 3, see Appendix 3 for a clear copy of the examination form, see Appendix 4 for a clear copy of the questionnaire form).
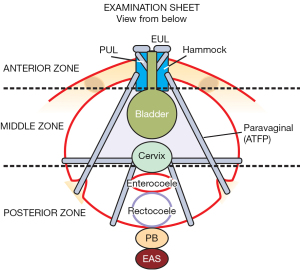
Typical examination findings of anatomical defects in the 3 zones
The Figures 3-5, which are generic figures, demonstrate what to look for in the vaginal examination; they are not necessarily related to the ITSQ patient example given previously.
Anterior zone examination for PUL damage
PUL laxity, the main cause of SUI, cannot be visually confirmed by the presence of a prolapse. It can only be confirmed by the hemostat test (Figure 6).
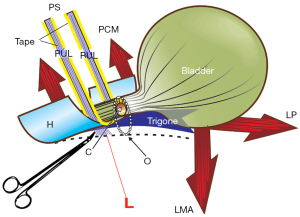
The patient is asked to cough; if urine loss during coughing is prevented by a hemostat gently pressed upward immediately behind the pubic bone, in the position of the midurethra, a diagnosis of lax PUL can be made (Figure 6) (see Video S4). This is recorded along with findings for vaginal hammock and the EUL.
Unilateral hemostat support, immediately behind the symphysis at the origins of the PUL, prevents the causative elongation of the PUL to “L”, and so controls urine loss on coughing (Video S4).
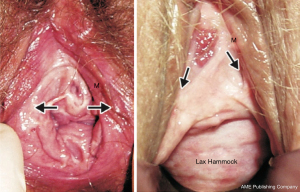
Anterior zone examination for EUL damage, and hammock laxity
The function of these structures is to seal the urethra. Laxity (Figure 7, right) needs to be repaired otherwise patients may say that SUI is cured but they still leak small amounts of urine, feeling like a bubble escaping.
Middle zone examination for transverse cardinal (CL) defect cystocele
A transverse cardinal defect cystocele usually has rugae and prolapses lateral to the cervix (Figure 8A).
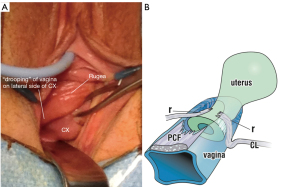
Pathogenesis of transverse defect cystocele
The CL attachment onto the anterior cervix is stretched or torn and prolapses downwards, lateral to the cervix, taking the prolapsed vagina with it (Figure 8A). The vaginal prolapse is caused by tearing of the pubocervical fascial (PCF) attachment to the CL (Figure 8B). The pubocervical layer and the underlying vagina descend downwards like a trapdoor as a transverse defect or “high” cystocele. The examination results are recorded in the examination sheet.
Clinical test for CL dislocation cause of transverse defect cystocele
With two Allis forceps, very gently grasp each side of the prolapsed vagina, lateral to the cervix (Figure 8), and approximate them to the midline of the cervix. The cystocele disappears.
Caution: this test must be performed with extreme gentleness so as not to cause pain.
Middle zone examination for central cystocele
Characteristically, there is a large bulge with shiny vaginal epithelium, with no obvious rugae. Invariably, there is also a transverse defect (Figure 9, see curved arrows, “cervical ring”).
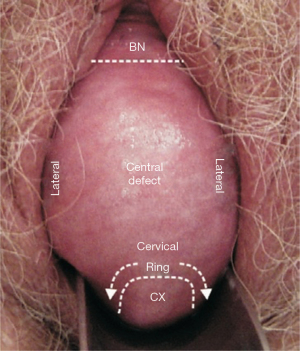
Pathogenesis of central defect is essentially unknown
Repair of dislocated puboccygeus muscles to cure a diverted urinary stream with a USling (5), cured a central cystocele. Repeated confirmation of cure of a central cystocele as in Figure 9, by a USling, by several surgeons, caused us to hypothesize that its pathogenesis may be pubococcygeus muscle dislocation from the symphysis.
Posterior zone examination for USL laxity
In Figures 9,10, uterine prolapse of 4th degree is evident. Figure 10 also shows that with a large degree of uterine prolapse, the CL must also be stretched equivalently. More difficult to confirm is minimal prolapse, 1st and 2nd degree, which often has the worst symptoms, and is usually only confirmed in the OR by pulling down the cervix. Clinical confirmation of such cases is by elevating the anterior vaginal wall with a speculum and asking the patient to strain down. Appearance of an enterocele confirms USL elongation. Not diagnosable by vaginal examination is the frequent co-occurrence of anterior rectal wall intussusception with uterine/apical prolapse which requires diagnosis by X-ray proctogram (6) or by ultrasound (see “Simulated operations” paper in this series).
Video S5 demonstrates that the CL plays a causative role equivalent to the USL, in 4th degree uterine prolapse, as indicated in Figure 10. Note in Video S5, how there was significant restoration of the uterine prolapse when Professor Sekiguchi tightened the CL TFS tape. Professor Sekiguchi’s restoration of uterine prolapse and cystocele by shortening and reinforcing the CL and USL (Figure 10), is, in fact, a more modern version of Fothergill’s Manchester Repair operation (Video S5 is by permission of Professor Sekiguchi).
Diagnosis of minimal USL laxity
In the outpatient clinic, lifting up the anterior vaginal wall with a speculum, and asking the patient to strain down hard can provoke an enterocele bulge in the posterior wall to confirm USL laxity.
Posterior zone examination for perineal body damage
There is a considerable bulge of the perineum on straining (Figure 11) (see Video S6). Rectal examination usually confirms that there is very little tissue between the vagina and the rectum.
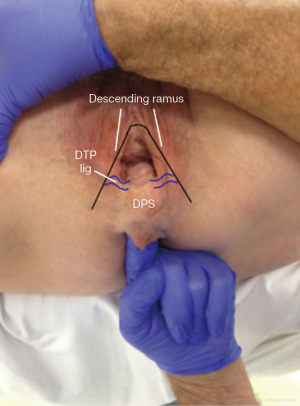
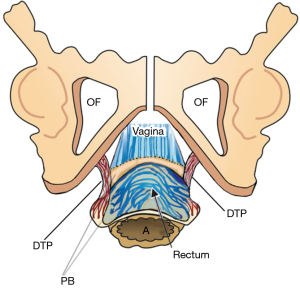
Pathogenesis of perineocele and descending perineal syndrome
Rectal examination confirms flattening and lateral displacement of the perineal bodies which are usually to be found below the ischial tuberosity. The cause is elongation of the deep transversus perinei (DTP) ligaments (Figure 12). The lateral displacement of these structures allows protrusion of the rectum into the central space which presents as a low rectocele or perineocele. Often the serosal layer of the rectum has been breached. This allows the rectal mucosa to spread laterally and to adhere to the vagina, DTP ligaments and even the pelvic bones. If the damage is sufficient, the perineal body itself descends as “descending perineal syndrome” (7,8).
How accurate was the algorithm in predicting examination findings?
Figure 13 compares the examination sheet findings (Figure 5) with the algorithm predictions from Figure 4, (both from the ITSQ patient example):
- Anterior zone prediction—SUI confirmed by hemostat supporting the PUL.
- Middle zone prediction—cystocele confirmed.
- Posterior zone prediction—uterine prolapse, enterocele, lax perineum confirmed.
What this means: a clinician experienced in the diagnostic system can figure out fairly accurately which ligaments may be causing patient problems purely from the symptoms, especially where there is minimal prolapse. For example, SUI = PUL damage; pain and nocturia = USL damage; bladder emptying/retention = USL or CL, often both.
Part IV of the flow chart—simulated operations
Though simulated operations follow vaginal examination in the flow chart (Figure 1), they are actually performed during the vaginal examination. Specific structures such as PUL and USL, and bladder base, are mechanically supported and the change in symptoms of stress incontinence, urge and pain are observed. Many simulated operations are possible, and these are presented in detail in this Annals of Translational Medicine series in the paper, “Simulated Operations apply mechanical support to structures to confirm symptom causation”. Simulated operations for PUL and USL, are presented here, as they have become part of a standard vaginal assessment.
Simulated operation for stress and mixed incontinence
A hemostat applied behind the symphysis mechanically supports a weak PUL and prevents PUL elongation on coughing or straining, as in Figure 6. If it prevents urine loss on coughing, it predicts a midurethral sling should cure or improve SUI. If the hemostat test reduces urge also, it can be predicted that the urge component of “mixed incontinence” (stress plus urge) will also be cured or improved in 50% of such women.
Confirming urinary urge and CPP objectively:speculum test (simulated operation)
The speculum test (Figure 14) is a simple and available test for determining USL pathogenesis of CPP and urgency.
Confirming nocturia objectively
Shkarupa et al. (9) successfully used a gauze roll inserted during the day and overnight to test eligibility of patients with OAB symptoms for CL/USL plication (9) (see Appendix 5 for further references which surgically validate the predictive value of the diagnostic system).
Adding objective testing to clinical assessment
Objective investigative assessment is essentially the same as the four-part clinical assessment but adds more investigative information and objective tests. Examples in Figure 15, are patient diaries, pad tests (10) 2D or 3D transperineal ultrasound (11), specific urodynamic tests such as “interventional urodynamics” which mechanically support a ligament during urodynamic testing while observing the changes in the graphs (12,13). Such objective testing is standard practice in many tertiary academic centres. In some cases, such as TVS, tests such as 2D ultrasound monitoring during straining, add a substantial layer of diagnostic certainty.
With the caveat of variability inherent in all short objective tests such as diaries, urodynamics, pad tests, the protocols detailed in Figure 15 could be used as a starting base for a future “evidence-based” artificial intelligence (AI) diagnostic system. For example, the prototype Bayesian Network learning model (Figure 6), is potentially configurable for an individual demographic. Figure 15 is a more expanded assessment tool than the clinical Structured Assessment System (1) (Figure 1).
Future directions: AI/Bayesian network learning
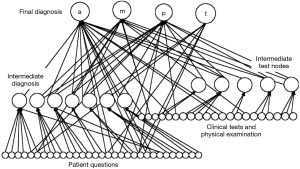
The Decision Tree Diagnostic System (Figure 16), contains percentage probabilities for specific input parameters. The decision tree worked accurately in the specialist referral environment where it was created. So as the decision could be more generally applied to other clinics which may have a different patient clientele, a machine learning system based on Bayesian networks which could be trained for a different environment and different clientele was developed by Curtin University School of Engineering, Perth, Western Australia, working with P.P. from Royal Perth Hospital (14). Matlab and the BN Toolbox were used to build the Bayesian networks. The Bayesian networks were trained using a majority of the data samples and then tested on the balance, ensuring that the test data was unseen by the network during training (14). Tests were also done using the training data. The results obtained by querying the network, for any individual case, were compared with the diagnosis obtained from the human expert (Peter Petros). These comparisons formed the basis of the evaluation of the system. In most cases, Bayesian networks were found to be at least as accurate as using Decision Trees, for example, Figure 16. An advantage of using Bayesian networks is that the accuracy, specificity and sensitivity will improve as the number of test cases available for training increases. In contrast, using Decision Trees is a relatively static process that is not easy to enhance incrementally.
Problems and some possible future directions
Each city, suburb, medical practice will have a different mix of patients. It is envisaged that a Bayesian-based learning machine will be able to train the diagnostic system to be more specific for a location, and, therefore, more accurate.
Conclusions
The ITP has a 25-year track record of curing or improving bladder/bowel/pain symptoms when it can be demonstrated they may be caused by ligament laxity, even when prolapse is minimal. Prior to undertaking any treatment, an accurate diagnostic protocol is required. The structured diagnostic flow chart is entirely clinical. It uses symptoms to diagnose anatomical defects, vaginal examination to confirm damage, and simulated operations to validate that a specific ligament is causing a specific symptom.
Acknowledgments
We would like to express our thanks to Vani Bardetta for her proofreading and administrative services for this article.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the International Society for Pelviperineology for the series “Integral Theory Paradigm” published in Annals of Translational Medicine. Peter Petros (Editor) and Vani Bardetta (Assistant Editor) served as the unpaid Guest Editors of the series. The article has undergone external peer review.
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-23-1759/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-23-1759/coif). The series “Integral Theory Paradigm” was commissioned by the International Society for Pelviperineology without any funding or sponsorship. Peter Petros serves as an unpaid editorial board member of Annals of Translational Medicine from October 2022 to September 2024. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All clinical procedures described in this study were performed in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for the publication of this article and accompanying images. Human participation in the video was by patient permission on the basis it was deidentified.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Petros PE. Chapter 3, Diagnosis of Connective tissue damage. In: The female pelvic floor: function, dysfunction and management according to the Integral Theory. Heidelberg: Springer Verlag; 2010:81-103.
- Petros PE, Ulmsten UI. An integral theory and its method for the diagnosis and management of female urinary incontinence. Scand J Urol Nephrol Suppl 1993;153:1-93.
- Goeschen K, Gold DM. Surgical cure of chronic pelvic pain, associated bladder & bowel symptoms by posterior sling in 198 patients validates the Pescatori Iceberg principle of pelvic symptom co-occurrence. Pelviperineology 2017;36:84-8.
- Wagenlehner FM, Fröhlich O, Bschleipfer T, et al. The Integral Theory System Questionnaire: an anatomically directed questionnaire to determine pelvic floor dysfunctions in women. World J Urol 2014;32:769-81. [Crossref] [PubMed]
- Scheffler KU, Petros PE, Oliver W, et al. A hypothesis for urinary stream divergence in the female: unilateral dislocation of the pubovisceral muscle. Pelviperineology 2014;33:10-3.
- Abendstein B, Brugger BA, Furtschegger A, et al. Role of the uterosacral ligaments in the causation of rectal intussusception, abnormal bowel emptying, and fecal incontinence-a prospective study. Pelviperineology 2008;27:118-21.
- Wagenlehner FME, Del Amo EGA, Santoro GA, et al. Live anatomy of the perineal body in patients with third-degree rectocele. Colorectal Dis 2013;15:1416-22. [Crossref] [PubMed]
- Petros PEP, Inoue H. Transvaginal perineal body repair for low rectocoele. Tech Coloproctol 2013;17:449-54. [Crossref] [PubMed]
- Shkarupa D, Zaytseva A, Kubin N, et al. Native tissue repair of cardinal/uterosacral ligaments cures overactive bladder and prolapse, but only in pre-menopausal women. Cent European J Urol 2021;74:372-8. [Crossref] [PubMed]
- Petros PE, Ulmsten U. An analysis of rapid pad testing and the history for the diagnosis of stress incontinence. Acta Obstet Gynecol Scand 1992;71:529-36. [Crossref] [PubMed]
- Petros PE, Von Konsky B. Anchoring the midurethra restores bladder neck anatomy and continence. Lancet 1999;354:997-8. [Crossref] [PubMed]
- Petros PE. Changes in bladder neck geometry and closure pressure following midurethral anchoring suggest a musculo-elastic mechanism activates closure. Neurourol Urodyn 2003;22:191-7. [Crossref] [PubMed]
- Petros PE. Detrusor instability and low compliance may represent different levels of disturbance in peripheral feedback control of the micturition reflex. Neurourol Urodyn 1999;18:81-91. [Crossref] [PubMed]
- Hunt M, von Konsky B, Venkatesh S, et al. Bayesian networks and decision trees in the diagnosis of female urinary incontinence. Engineering in Medicine and Biology Society 2000. Proceedings of the 22nd Annual International Conference of the IEEE 2000;1:551-4.


