
A brief physiology and pathophysiology of the anorectum based on the Integral Theory paradigm
Highlight box
Key findings
• Four reflex muscles forces open and close the anorectum. Dysfunction is mainly caused by pubourethral ligament, uterosacral ligament (USL) and perineal body (PB) laxity and can be cured or improved by repair thereof.
What is known and what is new?
• Pathogenesis of fecal incontinence (FI) and obstructive defecation syndrome (ODS) is said to be multifactorial.
• Binary cortico/peripheral control of bladder and bowel is virtually identical: pelvic muscles contract against pelvic ligaments to open and close the anorectum. Damaged ligaments are the main cause of anorectal dysfunctions, FI and ODS.
What is the implication, and what should change now?
• The pathogenesis of FI and ODS is almost identical and both can be cured or improved at the same time by USL and/or PB repair.
Introduction
The key points of the article are summarized in the video abstract (Video S1).
The remit of this review is confined to the experimental scientific works and surgeries of the Integral Theory paradigm (ITP). Anorectal function is reflex and binary, with cortical and peripheral components (1). The musculoelastic theory of anorectal function and dysfunction is part of the ITP (1). A full account of the original scientific work on which the colorectal part of the ITP is based, can be found in the 12 original experimental studies (see https://www.researchgate.net/publication/267778578_The_Musculo-Elastic_Theory_of_anorectal_function_and_dysfunction) (1).
Functional anorectal anatomy
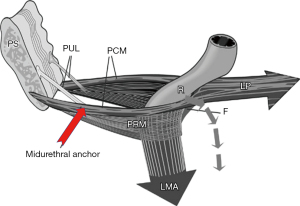
There are four main reflex pelvic muscles, pubococcygeus muscle (PCM), levator plate (LP), conjoint longitudinal muscle of the anus (LMA), and puborectalis muscle (PRM). The PCM and LP contract against pubourethral ligaments (PULs); the LMA and LP contract against the uterosacral ligaments (USLs); the PRM contracts only against the pubic symphysis. These four muscles contribute to the binary control of anorectal functions (Figure 1).
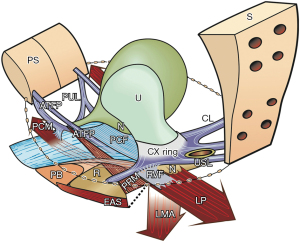
Four reflex striated pelvic muscles open and close the anorectum and control fecal urgency
With reference to Figure 1, the same three reflex forces, PCM, LP, LMA, which control bladder continence and evacuation, perform the same functions for the bowel, and contract against the PUL and USL:
- To close the anorectum for continence when the PRM contracts forwards (Figure 1, Video S2).
- To open the anorectum prior to evacuation when the PRM relaxes (Figure 1, broken lines; Videos S3,S4).
- To stretch the rectum in opposite directions to support the anorectal stretch receptors “N” to prevent premature activation of the defecation reflex (fecal urgency) (Figure 1).
Role of the perineal body (PB)
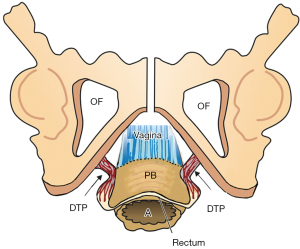
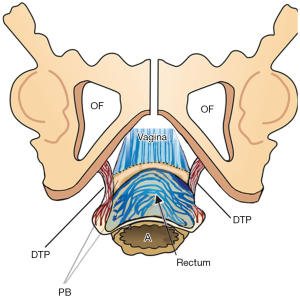
The PB attaches to the distal vagina, anorectum and external anal sphincter (EAS) and acts as an anatomical support for the distal vagina, rectovaginal fascia and anus (Figure 1). The PB is suspended from the descending pubic rami by the deep transversus perinei ligaments (DTPs) (Figure 2). DTPs are about 4 cm long, 7 mm in diameter, and attach behind the rami exactly between the upper 2/3 and lower 1/3. The PCM contracts forwards against the PB to tension the anterior rectal wall and anus during closure and defecation. The PB is an insertion point for the EAS.
Anorectal and bladder control
Anorectal and bladder control are very similar (1) (Figure 3). Both are binary controlled, and are regulated by two cortically controlled reflexes, either closed (closure reflex) or open (micturition, defecation reflexes). These reflexes comprise cortical and peripheral components (striated pelvic muscle/ligaments) and are mutually exclusive. The binary control system works like an electric switch which controls two different circuits, one at a time, either closed or open. Though control is autonomic, the cortex exerts voluntary control over both closure and evacuation reflexes (2,3).
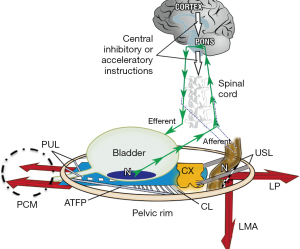
With reference to Figure 3, the binary cortico/peripheral control of bladder and bowel is virtually identical. Afferent fibres from stretch receptors “N” in the bladder and bowel transmit afferent nerve emptying impulses to the cortex which interprets them as “fullness”. By reflexly stretching the bladder and bowel bidirectionally (large arrows), the muscles tension the underlying supports of the stretch receptors “N” of each organ to prevent them from firing off emptying impulses prematurely, thereby controlling inappropriate activation of the micturition and defecation reflexes, which is sensed by the cortex as urgency. If convenient to empty, the closure reflex shuts down, and the emptying reflexes (micturition and defecation) are activated. The posterior walls of the urethra and anorectum are actively pulled open (broken lines) by the LP/LMA immediately prior to evacuation. This external opening exponentially decreases resistance to urine or fecal flow, thereby facilitating evacuation (4).
The mechanics of normal anorectal closure and opening
With reference to Figure 1 and Video S2, for anorectal closure, the PRM contracts forward against the symphysis; the LP contracts backward against the PUL to tension the rectum; the LMA contracts downward against the USL to rotate the rectum around a contracted PRM to close the anorectal angle.
For defecation, in Figure 1, (broken lines behind rectum) and Video S3, the PRM relaxes; the LP/LMA pull the posterior rectal wall backward/downward to open out the anorectal angle; the PCM contracts forward to stiffen the anterior rectal wall; the lateral walls of the rectum are pulled outwards, most likely by contraction of the iliococcygeus muscle via its insertion into the lateral wall of PCM (Video S4). The rectum opens radially and appears to shorten by downward shrinking of the walls, as demonstrated in both sagittal and anterior-posterior views (Video S4). These opening actions exponentially decrease the internal anal resistance to the passage of feces to facilitate fecal evacuation (4,5).
Dysfunction
Damage to any part of the binary system may interfere with the binary control of all its functions to cause anorectal (and bladder) dysfunctions (Figure 3). For example, these include damage to the facilitatory or inhibitory centres of the brain or spinal cord; to the afferent or efferent nerves (for example by multiple sclerosis); to any part of the peripheral control system, be it muscle or ligament or the organ itself; by infection or local pressure by cancer to the stretch receptors “N” or by external pressure on “N” by cervical fibroids for the bladder, or rectal mucosal prolapse for the anorectum.
Fecal incontinence (FI)
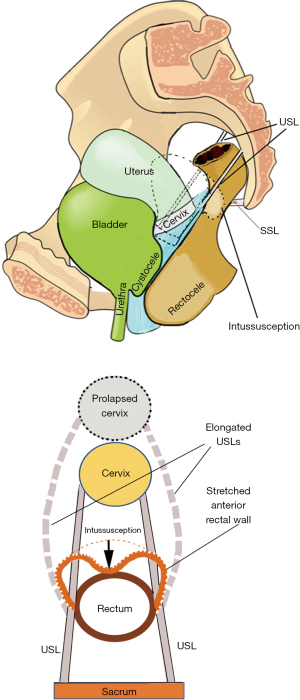
In an individual woman, FI may have many causes. The scope of this review is limited to the role of loose ligaments in FI, as indicated in the diagnostic algorithm (Figure 4). Note how FI can be caused by both PUL and USL weakness. The most common cause of FI is USL weakness or laxity. This type of FI is associated with uterine/apical prolapse (often minimal) and other posterior zone symptoms (pain and bladder symptoms) (Figure 4, 3rd column). Other co-occurring manifestations of USL laxity are obstructive defecation, and sometimes, anterior rectal wall intussusception (Figure 5). A not so well recognized cause of FI is PUL weakness, which co-occurs with stress urinary incontinence (SUI), which is known as “double incontinence”. The pathogenesis of “double incontinence” is explained by reference to Figure 6: a weak or loose PUL anchoring point may weaken the backward contraction of the LP and its ability to stretch the rectum into the semirigid structure required for it to be pulled down and “kinked” like a garden hose around a contracted PRM. Any looseness in the PUL can result in FI as well as SUI. A hemostat applied at the midurethra on one side as in Figure 6, will control both urinary and FI seen on coughing (7). Both the SUI and FI of “double incontinence” may be cured by a midurethral sling (6).
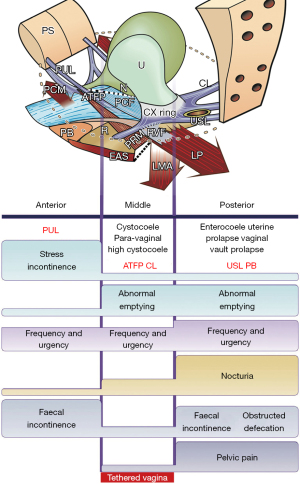
Obstructive defecation syndrome (ODS)
ODS is a form of constipation. With reference to Figure 1, a loose USL anchoring point may weaken pelvic muscle ability to open the anorectal angle (broken lines), so that the rectum contracts against an unopened anus. This is perceived as ODS. Both FI and ODS can co-occur with anterior rectal wall intussusception (Figure 6), and both can be cured by a posterior sling inserted to reinforce the weakened USLs (8).
How uterine prolapse may cause anterior rectal wall intussusception
The USLs are loosely attached to the lateral walls of the rectum with a number of fine small narrow ligaments (9). With reference to Figure 5 (bottom figure), childbirth may stretch and elongate the USLs, dragging the anterior rectal wall forwards, loosening it, so it prolapses inwards as an intussusception. Figure 5 (bottom figure) shows how the attachment of USLs to the lateral wall of the rectum drags the more distensile lateral wall with them; as the USLs lengthen, they splay laterally. The anterior rectal wall, therefore, also elongates laterally, weakening it structurally. Its collagen concentration lessens and the anterior rectal wall invaginates to cause intussusception.
Pathogenesis of perineocele and descending perineal syndrome (DPS)
The pressure of the fetal head exiting the vagina stretches the PB laterally and downwards. The DTPs are stretched and elongated (Figure 7). Pressure on the anterior rectal wall stretches it laterally and often splits its serosal and muscular layers, leaving the thin anal mucosa exposed and adherent to the posterior vaginal wall which now bulges forward to cause perineocele (low rectocele) (10). If the PB and DTP are sufficiently stretched, the perineum may descend as “DPS”.
Anorectal muscle vectors require firm PUL and USL for balanced closure and defecation
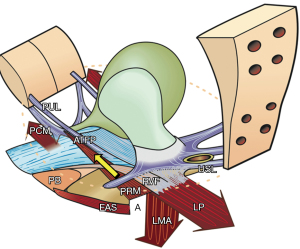
The four opposite striated muscle forces (Figure 8, large arrows), are always in balance. The forward vectors (PCM, PRM) and backward vectors (LP/LMA) act externally on the anorectum to facilitate normal closure and opening of the anorectal angle “A” (Figure 8). The PRM surrounds the rectum, but it is not attached to it. During closure, the PRM, which lies below the LP, contracts forward to pull the posterior wall of the rectum upwards and forwards. The backward muscle forces (LP/LMA) contract against competent USLs, to stretch the rectum down and around the PRM to close it in a “kinking” action, which also narrows the anorectal angle “A” (Figure 8). The PCM contracts forwards against the PUL during anorectal closure to steady the anterior rectal wall. During defecation, the PRM relaxes, the LP/LMA open out the posterior rectal wall enlarging the anorectal angle “A” (Figure 8). The PCM continues to contract during defecation; its role is to stretch the PB and anterior wall of the anus forwards to enlarge the diameter of the anal tube (11).
Imbalance of forward and backward forces may cause anorectal dysfunction
The anorectal angle “A” (Figure 8) is formed by the balanced backward/downward contraction of the LP/LMA against forward contraction of (mainly) the PRM. USL laxity will weaken LP/LMA forces which contract against USL. In relative terms, the PRM contracts more strongly than the LP/LMA; consequently, the directional muscle system (Figure 8, 3 large arrows) becomes unbalanced, and the PRM indents the posterior rectal wall, giving the impression of “paradoxical contraction” or “spasm” of the PRM as seen with defecating proctograms and ultrasound examinations (Figure 9). Figure 10 demonstrates how repair of the PUL and USL restored the anatomy both at rest and on straining, and also restored the function (see case report below).
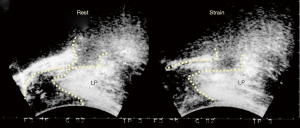
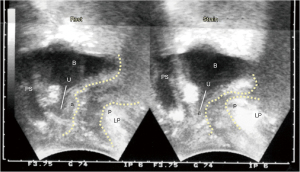
Ultrasound monitored loss of pelvic muscle equilibrium cured by PUL & USL repair
Experimental study No. 6: reused from (11). Copyright 2008, with permission from Pelviperineology.
The patient, 49 years old, parity 3, presented with 2nd degree prolapse, difficulty with defecation, FI, SUI, nocturia and chronic pelvic pain (1). Preoperative defecating proctogram demonstrated an acute anorectal angle similar to that of the preoperative ultrasound (Figure 9). There was no rectocele or rectal intussusception. PUL and USL sling repairs cured or substantially improved both bladder and bowel symptoms; also restored was the appearance of normal anatomy on straining in the transperineal ultrasound (Figure 10) which was attributed to restoration of the balance of the four directional forces from Figure 8. With reference to Figure 9, the indentation of the posterior wall of the rectum, both at rest and on straining, is consistent with the inability of the LP/LMA to balance the forward contraction of the PRM in Figure 8. On comparing Figure 9 to Figure 10, following USL repair (again with reference to Figure 8), the LP/LMA could now stretch the rectum backwards and downwards against USLs, in a rotating motion, to close (“kink”) the anorectal angle. At the same time, the PRM contracts forwards to provide a firm point for rotation of the rectum, and the PCM contracts forward to stabilize the anterior wall of the anus. A firm USL is required for the LP/LMA to exert maximum contractile force, and a firm PUL for PCM. The PRM contracts directly against the symphysis, so its contractile force is not affected by any ligament damage.
Surgical validation of USL causation of bladder/bowel dysfunctions
In addition to cure of several bladder dysfunctions (urge, frequency, nocturia, emptying) application of USL slings achieved cure rates ranging between 65% and 85% for dysfunctions of anorectal closure (“FI”) and evacuation (“ODS”) (12-19). If women with either FI or ODS have co-occurring bladder or pain symptoms (Figure 4, posterior zone), there is a high probability of surgical cure of both FI and ODS with USL repair, as demonstrated (12-19).
Prima facie, these results go some way towards validating ligament pathogenesis for these conditions. According to the Theory (1), however, it is the pelvic muscles which close and evacuate the anorectum (albeit by contracting against ligaments). Therefore, it is possible that failure to cure FI and ODS may have been due to pelvic muscle damage (20), which raised the question, which is the main pathogenic factor, damaged ligaments, or damaged muscles? This question was answered indirectly by a blinded biopsy muscle study of 47 women having a midurethral sling for SUI. The histology demonstrated that the vast majority had muscle injury, yet 90% were cured of their SUI the next day (21). Further evidence can be deduced from the anatomical pathway hypothesized by the anorectal theory for FI and ODS (1). This is graphically represented in Figure 11. The USLs, the insertion point of the LP/LMA, are loose and the uterus has prolapsed. The LP and LMA also effectively lengthen by “E”, “A” (Figure 11). Consequently, their contractility diminishes (“B”, Figure 11), as the striated muscle sarcomere requires a firm insertion point for optimum contractility (22).
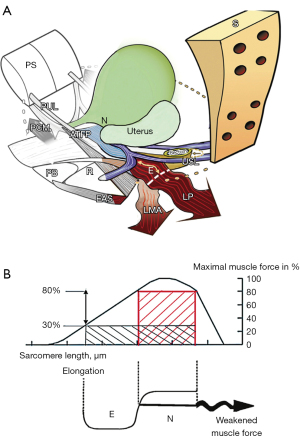
Conclusions
Within the context of the ITP, bladder and bowel dysfunctions co-occur mainly because of similar ligamentous pathogenesis. Both can be cured or improved at the same time by appropriate ligament repair. Though high cure rates have been achieved with ligament repair for either dysfunction, consideration must be given to the possible effects of nerve and muscle damage in an individual patient, which cannot be diagnosed and, therefore, cannot be surgically repaired.
Acknowledgments
We would like to express our thanks to Editors Professor Peter Petros and Vani Bardetta for their tremendous help in designing and modifying the article.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the International Society for Pelviperineology for the series “Integral Theory Paradigm” published in Annals of Translational Medicine. Peter Petros (Editor) and Vani Bardetta (Assistant Editor) served as the unpaid Guest Editors of the series. The article has undergone external peer review.
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-23-1883/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-23-1883/coif). The series “Integral Theory Paradigm” was commissioned by the International Society for Pelviperineology without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All clinical procedures described in this study were performed in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for the publication of this article, accompanying images and videos. Human participation in the videos was by patients permission on the basis it was deidentified.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Petros P, Swash M. The Musculo-Elastic Theory of anorectal function and dysfunction. Pelviperineology 2008;27:89-93.
- Petros P, Quaghebeur J, Wyndaele JJ. Defining urge as an uncontrolled micturition explains pathogenesis, informs cure and helps solve the burgeoning OAB crisis. Neurourol Urodyn 2022;41:1281-92. [Crossref] [PubMed]
- Petros P, Swash M, Bush M, et al. Defecation 1: Testing a hypothesis for pelvic striated muscle action to open the anorectum. Tech Coloproctol 2012;16:437-43. [Crossref] [PubMed]
- Bush M, Petros P, Swash M, et al. Defecation 2: Internal anorectal resistance is a critical factor in defecatory disorders. Tech Coloproctol 2012;16:445-50. [Crossref] [PubMed]
- Farag A. Use of the Hagen-Poiseuille law: a new mathematical approach for the integration and evaluation of anorectal physiological testing in patients with faecal incontinence and pelvic dyschezia and in normal controls. Eur Surg Res 1998;30:279-89. [Crossref] [PubMed]
- Hocking IW. Experimental study No. 9: Double incontinence, urinary and fecal, cured by surgical reinforcement of the pubourethral ligaments. Pelviperineology 2008;27:110.
- Petros PE, Swash M. Experimental Study No. 2. A direct test for the role of the pubourethral ligament in anorectal closure. Pelviperineology 2008;27:98.
- Abendstein B, Brugger C, Furtschegger A, et al. Study No. 12: Role of the uterosacral ligaments in the causation of rectal intussusception, abnormal bowel emptying, and fecal incontinence. A prospective study. Pelviperineology 2008;27:118-21.
- Petros PEP. The biomechanics of uterine prolapse impact rectal intussusception, ODS and surgical restoration. Tech Coloproctol 2022;26:161-2. [Crossref] [PubMed]
- Petros PE. Tissue Fixation System Perineal Body Repair: A Minimally Invasive Method for Repair of Descending Perineal Syndrome. Dis Colon Rectum 2016;59:e455. [Crossref] [PubMed]
- Petros PE, Swash M. Correction of abnormal geometry and dysfunction by suspensory ligament reconstruction gives insights into mechanisms for anorectal angle formation. Pelviperineology 2008;27:103-4.
- Wagenlehner F, Muller-Funogea I, Perletti G, et al. Vaginal apical prolapse repair using two different sling techniques improves chronic pelvic pain, urgency and nocturia – a multicentre study of 1420 patients. Pelviperineology 2016;35:99-104.
- Inoue H, Kohata Y, Fukuda T, et al. Repair of damaged ligaments with tissue fixation system minisling is sufficient to cure major prolapse in all three compartments: 5-year data. J Obstet Gynaecol Res 2017;43:1570-7. [Crossref] [PubMed]
- Inoue H, Nakamura R, Sekiguchi Y, et al. Tissue Fixation System ligament repair cures major pelvic organ prolapse in ageing women with minimal complications - a 10-year Japanese experience in 960 women. Cent European J Urol 2021;74:552-62. [Crossref] [PubMed]
- Goeschen K, Gold DM. Surgical cure of chronic pelvic pain, associated bladder & bowel symptoms by posterior sling in 198 patients validates the Pescatori Iceberg principle of pelvic symptom co-occurrence. Pelviperineology 2017;36:84-8.
- Petros P, Richardson PA. TFS posterior sling improves overactive bladder, pelvic pain and abnormal emptying, even with minor prolapse. A prospective urodynamic study. Pelviperineology 2010;29:52-55.
- Petros P, Abendstein B, Swash M. Retention of urine in women is alleviated by uterosacral ligament repair: implications for Fowler's syndrome. Cent European J Urol 2018;71:436-43. [Crossref] [PubMed]
- Liedl B, Inoue H, Sekiguchi Y, et al. Is overactive bladder in the female surgically curable by ligament repair? Cent European J Urol 2017;70:53-9. [Crossref] [PubMed]
- Himmler M, Göttl K, Witczak M, et al. The impact of transvaginal, mesh-augmented level one apical repair on anorectal dysfunction due to pelvic organ prolapse. Int Urogynecol J 2022;33:3261-73. [Crossref] [PubMed]
- Swash M, Snooks SJ, Henry MM. Unifying concept of pelvic floor disorders and incontinence. J R Soc Med 1985;78:906-11. [Crossref] [PubMed]
- Petros P, Swash M, Kakulas B. Experimental Study No. 8. Stress urinary incontinence results from muscle weakness and ligamentous laxity in the pelvic floor. Pelviperineology 2008;27:107-9.
- Gordon AM, Huxley AF, Julian FJ. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol 1966;184:170-92. [Crossref] [PubMed]


